|
 |
- Search
| Psychiatry Investig > Volume 8(2); 2011 > Article |
Abstract
Objective
Our aim was to evaluate the changes in blood glucose control and lipid profiles after 2-months of smoking cessation in healthy males.
Methods
Smoking abstinence was evaluated through self-report and urine cotinine levels. 12 individuals who succeeded in quitting smoking were analyzed. Fasting values of glucose and insulin were used to estimate the β-cell activity and insulin resistance was evaluated using the Homeostasis Model Assessment (HOMA) and Quantitative Insulin Sensitivity Check Index (QUICKI).
Results
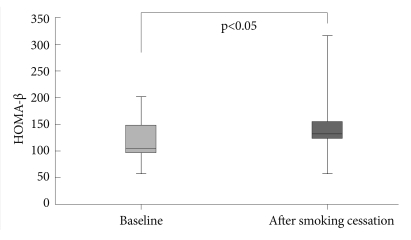
The data showed that the subjects had a significant increase in weight, body mass index and fasting plasma glucose levels after smoking cessation. The HOMA-Insulin Resistance and the HOMA β-cell function increased significantly (p=0.005, p=0.047 respectively). The QUICKI showed a significant decrease (p=0.005). In addition, the low-density lipoprotein cholesterol levels decreased significantly (p=0.028); however, changes in the high-density lipoprotein cholesterol, the triglyceride and total cholesterol levels were not significant (p=0.284, p=0.445 respectively).
Conclusion
During the initial stage of smoking abstinence, insulin resistance increased and insulin sensitivity decreased due to elevated body weight and fat composition. Therefore, it is important to educate individuals that stop smoking about the necessity of weight control during smoking cessation programs.
Smoking is known to negatively impact human health. Carcinogenic processes, vasomotor dysfunction, impaired endothelial-dependent vasodilatation, and the modification of lipid profiles are included in these adverse effects.1,2 Cigarette smoking has been clearly identified as an independent, major risk factor for atherosclerosis and coronary heart disease (CHD)3-5 and myocardial infarction.6 There is significant epidemiological evidence that has shown that smoking cessation reduces morbidity and mortality from CHD.7,8
Although the underlying mechanism is not clear, Kong et al.9 suggested that the consequent increase in hepatic lipase activity associated with smoking may in turn lead to increased lipid accumulation in the arterial wall and may represent one mechanism whereby smoking further increases the risk of cardiovascular disease in Type 2 diabetic subjects.
Smoking has also been shown to be associated with insulin resistance in both non-diabetic10 and Type 2 diabetic subjects,11 with impaired oral fat tolerance,12 impaired intravascular lipolysis13 and dyslipidemia; these patients are characterized by an atherogenic lipoprotein phenotype, with increased triglyceride (TG) and low high density lipoprotein cholesterol (HDL-C) concentrations, and high levels of low density lipoprotein cholesterol (LDL-C).14,15
Whether smokers have worse lipid profiles than non-smokers is controversial. A meta-analysis of 54 cross-sectional studies with respect to the association of lipid profiles and cigarette smoking status revealed that smokers had higher serum concentrations of total cholesterol (TC) (3.0%), TG (9.1%), very-low-density lipoprotein cholesterol (10.4%), and LDL-C (1.7%), and lower serum concentrations of HDL-C (5.7%) and apolipoprotein A1 (4.2%) than nonsmokers. In addition, a meta-an-alysis of 27 prospective studies, on the effects of smoking cessation on lipid profiles revealed that smoking cessation significantly increased the level of HDL-C but not of TC, LDL-C, or TG.16 However, there is limited information available regarding the effect of smoking cessation on blood glucose control and lipid profiles. Therefore, the changes in blood glucose control, lipid profiles, and insulin resistance-associated hormones both before and after 2-months of smoking cessation in Korean male smokers were examined.
Twenty male volunteers 28-52 years (mean age of 39 years) were enrolled from among the hospital staff at the Bucheon St. Mary's hospital at The Catholic University of Korea via advertisements in the hospital newspapers and website. All of the participants fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for nicotine dependence. The DSM-IV-TR diagnoses were determined by two board-certified psychiatrists. All participants abstained from illegal drugs and other medications and none had mental or physical disorders except for nicotine dependence. None of the healthy volunteers had a family history of psychiatric illness or medical problems. As a part of the initial clinical evaluation, all of the smokers were asked to complete baseline questionnaires requesting detailed information regarding demographics and smoking-related clinical variables. The severity of nicotine dependence was evaluated by the Fagerström Test for Nicotine Dependence (FTND).17 In order to minimize the influence of alcohol and caffeine on the lipid profile, all participants were instructed not to drink more than five standard alcoholic drinks a week and one cup of coffee per day, and not to change their eating habits during the study period. None of the participants were diabetic (fasting glucose <126 mg/dL) at baseline. Throughout the study period, all subjects participated in spontaneous smoking cessation without taking medication or nicotine replacement therapy.
During the study period, smoking abstinence was evaluated through self-report and urine cotinine levels. The self-report was assessed based on the responses to the question Have you quit smoking since you enrolled in this study? The urine cotinine levels of all subjects were monitored three times: before cessation, one month after, and two months after smoking cessation. Urinary samples were obtained without prior warning. The urine cotinine levels were measured by a urinary cotinine immunoassay dipstick (Nicometer, Jant Pharmaceutical, CA, USA). Among 20 participants, 12 smokers succeeded in quitting smoking (validated by a urine cotinine level ≤40 ng/mL) and eight smokers failed. Therefore, only the 12 successful individuals were analyzed.
Written informed consent was obtained from each subject after they had been given a complete explanation of the study. The protocol was approved by the Bucheon St. Mary's Hospital ethics committee of the Catholic University of Korea and all procedures conformed to the Helsinki Declaration of 1975, as revised in 1983.
Blood was drawn from the participants twice, at baseline and 2 months after smoking cessation. At 7:00 a.m., following an overnight fast, a sample of blood was collected from all subjects to measure the plasma glucose, HDL-C, LDL-C, TC, TG, total protein and albumin levels. The plasma glucose, TC, TG, HDL-C, and LDL-C levels were analyzed using enzymatic techniques (Choongwae Pharma., CO., Seoul, Korea) with a Hitachi 7600-110 analyzer. Thyroid stimulating hormone (TSH) concentrations were analyzed by immunoradiometric assay using an IRMA-mat TSH (IZOTOP, The Institute of Isotopes Co., Ltd., Budapest, Hungary) with RIA-mat-280 equipment. Triiodothyronine (T3) levels were analyzed using RIA-mat-280 equipment with the RIA-mat-T3 (Diasorin, Stillwater, USA). Free thyroxine (free T4) levels were analyzed using RIA-mat-280 equipment with the RIA-FT4 (Immunotech, Radiova, Prague, Czech Republic). Growth hormone levels were analyzed using immunoradiometric assay techniques (Daiichi,Tokyo, Japan) in a 1,470 wizard γ-counter. Somatomedin-C levels were analyzed by radioimmunoassay (BioSource Europe S.A., Belgium) in a 1,470 wizard γ-counter. Adrenocorticotropic hormone (ACTH) levels were measured by immunoradiometric assay, mixing samples with ELSA-ACTH (CIS bio international, Gif-sur-Yvette Cedex, France) in a 1,470 wizard γ-counter.
Insulin concentrations were analyzed by immunoradiometric assay (TFB, Tokyo, Japan). Plasma glucose levels were analyzed using a Roche/Hitachi Modular P analyzer by mixing samples with Gluco-quant Glucose/HK (Roche Diagnostics, Indianapolis, IN., USA). Epinephrine, norepinephrine and cortisol were assayed in duplicate by radioimmunoassay using commercial kits (BioSource Europe S.A., Belgium).
Body composition was analyzed using the Inbody 720 (Biospace. CO., Ltd., Seoul, Korea). All body composition parameters such as the body mass index (BMI), body fat mass, waist hip ratio and skeletal muscle mass were compared at baseline to measurements after 2-months of abstinence.
Using the fasting glucose and insulin data obtained, the Quantitative Insulin Sensitivity Check Index (QUICKI) and the Homeostasis Model Assessment-Insulin Resistance (HOMA-IR) were used to calculate insulin activity. Insulin secretion was assessed by the Homeostasis Model Assessment β-cell function (HOMA-β) from data obtained while in a fasting state.
The mean age of the subjects was 39.0±7.8, with a range of 28-52 years of age. The mean age that the subjects began smoking was 18.6±1.7 with a range of 17-22 years of age. The mean number of cigarettes consumed per day was 19.3±1.7. The mean score for the FTND was 2.8±1.7.
Body weight (p=0.029) and waist circumference (p=0.020) increased during the two months of smoking cessation. Body fat composition increased more than muscle (p=0.013) (Table 1).
LDL-C levels decreased significantly (p=0.028) after smoking cessation; however, changes in HDL-C, TC and TG were not significant (Table 1).
The data did not reveal any effects from short-term smoking abstinence on thyroid hormones; however, the T3 levels decreased (p=0.005) and the cortisol levels increased significantly (p=0.047) (Table 1).
Previous studies have reported that smoking is associated with increased blood glucose concentrations, insulin resistance and impaired insulin sensitivity.9,18-20 Blood glucose levels are affected by diet. However, smoking has been shown to have long-term effects on glucose homeostasis in men and women without diabetes, even after adjustment for dietary factors.21 Cigarette smoking and nicotine administration increase the circulating levels of antagonistic insulin hormones (i.e., catecholamines, cortisol, and growth gormone).22,23 It is possible that nicotine, via these mechanisms, impairs insulin sensitivity, directly and indirectly.24 Cessation of smoking increases insulin sensitivity and normalizes insulin resistance in subjects with and without diabetes, despite an increase in the BMI following smoking cessation.25,26
The results of this study showed that insulin resistance increased and insulin sensitivity decreased despite smoking cessation. This might have been due to weight gain, increased fat composition and increased cortisol after smoking cessation or the short duration of smoking cessation. After an extended time of smoking cessation, insulin resistance may decrease and insulin sensitivity may increase.27
In addition, the results of this study showed that during smoking cessation, insulin secretion increased more than during smoking. High beta cell activity, after smoking cessation, is not consistent with the findings of some prior studies.27,28 The likely explanation for this finding is the compensation of beta cells for insulin resistance.
Smoking was positively associated with LDL-C in males of European, but not of African descent.29 It was also reported, in a longitudinal observational study during early adulthood, that white male and female smokers had a larger increase in their LDL-C compared to nonsmokers, and black female smokers had an inverse association with the LDL-C levels.30 It was demonstrated, in a cross-sectional observational study, that the HDL-C levels were lower and the triglyceride levels were hig-her in female as well as male smokers compared to nonsmokers in the Japanese.31 However, smoking was not related to the levels of the total cholesterol or the LDL-C in Singapore.32
The biological mechanisms explaining the effect of smoking on lipid metabolism have not been fully elucidated. Some laboratories have demonstrated that hepatic lipase is increased in smokers,9 and others have demonstrated no difference between smokers and nonsmokers,33 and even decreased levels of hepatic lipase in smokers.34 Hepatic lipase has been shown to be activated in smokers, and the lectin:cholesterol acyl transferase activity has been shown to be unchanged35 or decreased33 compared to nonsmokers. Plasma cholesterol ester transfer protein activity has been shown to be marginally decreased in smokers in one study33 and increased in another.35 Plasma post-heparin lipoprotein lipase activity is unchanged in smokers and nonsmokers in some studies34,36 and was increased in smokers in another study.33 As these findings suggest, there are significant inconsistencies in the findings of studies.
Regardless of the conflicting study results, plasma enzymes appear to be involved in the metabolism of triglycerides and HDL-C, which are potentially affected by smoking. It is possible that the effect of smoking on these enzymes is dependent on numerous factors including: gender, age, genetic background, and the ethnicity of smokers. Other important factors include dietary habits, physical activity, and lifestyle as well as differences in public health awareness.31
There have been very few studies examining the effect of smoking cessation on lipid profiles. In addition, the reported study results have been inconsistent. A meta-analysis of 27 studies concluded that HDL-C was significantly increased but that the levels of TC, LDL-C, and TG did not change significantly after smoking cessation.16 Quitting smoking led to an increase in HDL-C but no change in the LDL-C.37 There was also an increase in the resistance of LDL-C to oxidation and not of the serum level of LDL-C after smoking cessation.38
Some possible explanations for the changes in the lipid profile caused by smoking cessation include the following. HDL-C normally promotes cholesterol clearance and decreases LDL-C oxidation.39 Since oxidized LDL-C, but not native LDL-C, is taken up by macrophages, via a scavenger receptor family, and thereby causes the formation of foam cells observed in atherosclerotic lesions, oxidative modification of LDL-C is thought to be the key process underlying the development of atherosclerosis.38 Smoking might lower the level of HDL by the following: 1) increasing the cholesteryl ester transfer protein; 2) reducing lecithin cholesterol acyltransferase activity; 3) affecting apo-1 synthesis; and 4) increasing TG.40 These unfavorable conditions (i.e., decreased HDL-C) may be restored to favorable levels by smoking cessation. Another mechanism by which smoking could reduce HDL-C, increase triglycerides, and increase plasminogen activator inhibitor-1 is through insulin resistance.41,42
In this study, LDL-C levels decreased after smoking cessation, suggesting that the beneficial effects of smoking cessation; however, changes in HDL-C and TC were not significant. The discrepancy between the levels of cholesterol in this study compared to previous studies may be due to the fact that subjects that stopped smoking included those with recent and long-term cessation, resulting in the confounding of short- and long-term changes in the cholesterol levels.
The limitations of this study include the following. First, the sample size was relatively small. Future studies will need to recruit more subjects in order to increase the statistical power of this research. Second, only males were recruited for this study, so the findings are not generalizable to women. Gender differences in smoking behaviors and metabolic brain responses to nicotine have been reported.43-45 Third, we analyzed 12 smokers succeeded in quitting smoking instead of nonsmokers or smokers who couldn't quit smoking as control group. Fourth, there are some confounding factors affecting the results. As mentioned earlier, there are many differences between smokers and non-smokers with regard to background characteristics, such as diet, exercise, obesity, and drinking alcohol.3,4,31 In addition, we did not evaluate the change of appetite during smoking abstinence so we could not exclude the effect of appetite on weight gain. Fifth, the duration of smoking cessation was only 2-months. A prolonged period of cigarette smoking cessation may be necessary to determine the effects of the change in blood glucose control and lipid profiles.5,25,26
Despite these limitations, the strength of the present study is that the findings can be used to guide clinicians and patients in smoking cessation. It is important to include education about weight control with diet and regular exercise (both aerobic and anaerobic) for blood glucose control in smoking cessation programs.
In conclusion, the results of this study showed that during the initial stage of smoking cessation, insulin resistance increased and insulin sensitivity decreased due to elevated body weight and fat composition. Therefore, weight control including diet control and physical exercise is necessary as part of smoking cessation programs. In the short-term, the positive effects of quitting smoking might not be immediately obvious. Therefore, further longitudinal studies including the control of the BMI, physical activity and other confounding risk factors such as medical history and alcohol consumption are needed to evaluate the effects of smoking cessation on physical and laboratory findings.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0016708). The Ministry of Education, Science and Technology had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper of publication.
References
1. Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ 1989;298:784-788. PMID: 2496857.



2. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 1993;88:2149-2155. PMID: 8222109.


3. Kannel WB. Update on the role of cigarette smoking in coronary artery disease. Am Heart J 1981;101:319-328. PMID: 7008566.


4. Kannel WB. Cigarettes, coronary occlusions, and myocardial infarction. JAMA 1981;246:871-872. PMID: 7253166.


6. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-952. PMID: 15364185.


7. Rosenberg L, Palmer JR, Shapiro S. Decline in the risk of myocardial infarction among women who stop smoking. N Engl J Med 1990;322:213-217. PMID: 2294448.


8. Sato I, Nishida M, Okita K, Nishijima H, Kojima S, Matsumura N, et al. Beneficial effect of stopping smoking on future cardiac events in male smokers with previous myocardial infarction. Jpn Circ J 1992;56:217-222. PMID: 1552649.


9. Kong C, Nimmo L, Elatrozy T, Anyaoku V, Hughes C, Robinson S, et al. Smoking is associated with increased hepatic lipase activity, insulin resistance, dyslipidaemia and early atherosclerosis in Type 2 diabetes. Atherosclerosis 2001;156:373-378. PMID: 11395034.


10. Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet 1992;339:1128-1130. PMID: 1349365.


11. Targher G, Alberiche M, Zenere MB, Bonadonna RC, Muggeo M, Bonora E. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1997;82:3619-3624. PMID: 9360516.


12. Elkeles RS, Khan SR, Chowdhury V, Swallow MB. Effects of smoking on oral fat tolerance and high density lipoprotein cholesterol. Clin Sci (Lond) 1983;65:669-672. PMID: 6627853.


13. Richmond W, Seviour PW, Teal TK, Elkeles RS. Impaired intravascular lipolysis with changes in concentrations of high density lipoprotein subclasses in young smokers. Br Med J (Clin Res Ed) 1987;295:246-247.



14. Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988;260:1917-1921. PMID: 3418853.


16. Maeda K, Noguchi Y, Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med 2003;37:283-290. PMID: 14507483.


17. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-1127. PMID: 1932883.


18. Sandberg H, Roman L, Zavodnick J, Kupers N. The effect of smoking on serum somatotropin, immunoreactive insulin and blood glucose levels of young adult males. J Pharmacol Exp Ther 1973;184:787-791. PMID: 4687238.

19. Janzon L, Berntorp K, Hanson M, Lindell SE, Trell E. Glucose tolerance and smoking: a population study of oral and intravenous glucose tolerance tests in middle-aged men. Diabetologia 1983;25:86-88. PMID: 6354814.


20. Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistan--potential link with the insulin resistance syndrome. J Intern Med 1993;233:327-332. PMID: 8463765.


21. Sargeant LA, Khaw KT, Bingham S, Day NE, Luben RN, Oakes S, et al. Cigarette smoking and glycaemia: the EPIC-Norfolk Study. European Prospective Investigation into Cancer. Int J Epidemiol 2001;30:547-554. PMID: 11416081.



22. Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation 1993;88:562-571. PMID: 8339419.


23. Lucini D, Bertocchi F, Malliani A, Pagani M. A controlled study of the autonomic changes produced by habitual cigarette smoking in healthy subjects. Cardiovasc Res 1996;31:633-639. PMID: 8689656.



24. Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis 2003;45:405-413. PMID: 12704597.


25. Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur J Clin Invest 1997;27:450-456. PMID: 9179554.


26. Mikhailidis DP, Papadakis JA, Ganotakis ES. Smoking, diabetes and hyperlipidaemia. J R Soc Health 1998;118:91-93. PMID: 10076642.


27. Daniel M, Cargo MD. Association between smoking, insulin resistance and beta cell function in a North-western First Nation. Diabet Med 2004;21:188-193. PMID: 14984456.


28. Henkin L, Zaccaro D, Haffner S, Karter A, Rewers M, Sholinsky P, et al. Cigarette smoking, environmental tobacco smoke exposure and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Ann Epidemiol 1999;9:290-296. PMID: 10976855.


29. Halfon ST, Green MS, Heiss G. Smoking status and lipid levels in adults of different ethnic origins: the Jerusalem Lipid Research Clinic Program. Int J Epidemiol 1984;13:177-183. PMID: 6735562.



30. Freedman DS, Srinivasan SR, Shear CL, Hunter SM, Croft JB, Webber LS, et al. Cigarette smoking initiation and longitudinal changes in serum lipids and lipoproteins in early adulthood: the Bogalusa Heart Study. Am J Epidemiol 1986;124:207-219. PMID: 3728437.



31. Kuzuya M, Ando F, Iguchi A, Shimokata H. Effect of smoking habit on age-related changes in serum lipids: a cross-sectional and longitudinal analysis in a large Japanese cohort. Atherosclerosis 2006;185:183-190. PMID: 16051249.


32. Hughes K, Choo M, Kuperan P, Ong CN, Aw TC. Cardiovascular risk factors in relation to cigarette smoking: a population-based survey among Asians in Singapore. Atherosclerosis 1998;137:253-258. PMID: 9622268.


33. Freeman DJ, Griffin BA, Murray E, Lindsay GM, Gaffney D, Packard CJ, et al. Smoking and plasma lipoproteins in man: effects on low density lipoprotein cholesterol levels and high density lipoprotein subfraction distribution. Eur J Clin Invest 1993;23:630-640. PMID: 8281981.


34. Zaratin AC, Quintao EC, Sposito AC, Nunes VS, Lottenberg AM, Morton RE, et al. Smoking prevents the intravascular remodeling of high-density lipoprotein particles: implications for reverse cholesterol transport. Metabolism 2004;53:858-862. PMID: 15254877.


35. Dullaart RP, Hoogenberg K, Dikkeschei BD, van Tol A. Higher plasma lipid transfer protein activities and unfavorable lipoprotein changes in cigarette-smoking men. Arterioscler Thromb 1994;14:1581-1585. PMID: 7918308.


36. Eliasson B, Mero N, Taskinen MR, Smith U. The insulin resistance syndrome and postprandial lipid intolerance in smokers. Atherosclerosis 1997;129:79-88. PMID: 9069521.



37. Gerace TA, Hollis J, Ockene JK, Svendsen K. Smoking cessation and change in diastolic blood pressure, body weight, and plasma lipids. MRFIT Research Group. Prev Med 1991;20:602-620. PMID: 1758841.


38. Sasaki A, Kondo K, Sakamoto Y, Kurata H, Itakura H, Ikeda Y. Smoking cessation increases the resistance of low-density lipoprotein to oxidation. Atherosclerosis 1997;130:109-111. PMID: 9126654.


39. Moffatt RJ, Chelland SA, Pecott DL, Stamford BA. Acute exposure to environmental tobacco smoke reduces HDL-C and HDL2-C. Prev Med 2004;38:637-641. PMID: 15066367.



40. Freeman DJ, Packard CJ. Smoking and plasma lipoprotein metabolism. Clin Sci (Lond) 1995;89:333-342. PMID: 7493432.


41. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev 1995;75:473-486. PMID: 7624391.


42. Reaven GM. Banting Iecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595-1607. PMID: 3056758.


43. Delfino RJ, Jamner LD, Whalen CK. Temporal analysis of the relationship of smoking behavior and urges to mood states in men versus women. Nicotine Tob Res 2001;3:235-248. PMID: 11506767.


44. Fallon JH, Keator DB, Mbogori J, Taylor D, Potkin SG. Gender: a major determinant of brain response to nicotine. Int J Neuropsychopharmacol 2005;8:17-26. PMID: 15579215.



45. Kim TS, Kim DJ, Lee H, Kim YK. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci Lett 2007;423:53-57. PMID: 17662528.


Table 1
Profile of participants before and after smoking cessation
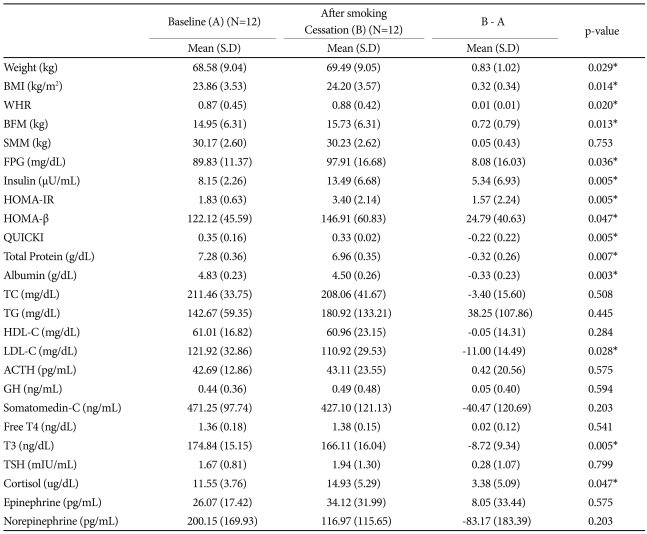
P-value was obtained by Wilcoxon signed rank test on differences. *p<0.05. BMI: body mass index, WHR: waist hip ratio, BFM: body fat mass, SMM: skeletal muscle mass, FPG: fasting plasma glucose, HOMA-IR: homeostasis model assessment-insulin resistance, HOMA-β: homeostasis model assessment β-cell function, QUICKI: quantitative insulin sensitivity check index, TC: total cholesterol, TG: triglyceride, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, ACTH: adreno cortico tropic hormone, GH: growth hormone, Free T4: free thyroxine, T3: thyronine, TSH: thyroid-stimulating hormone