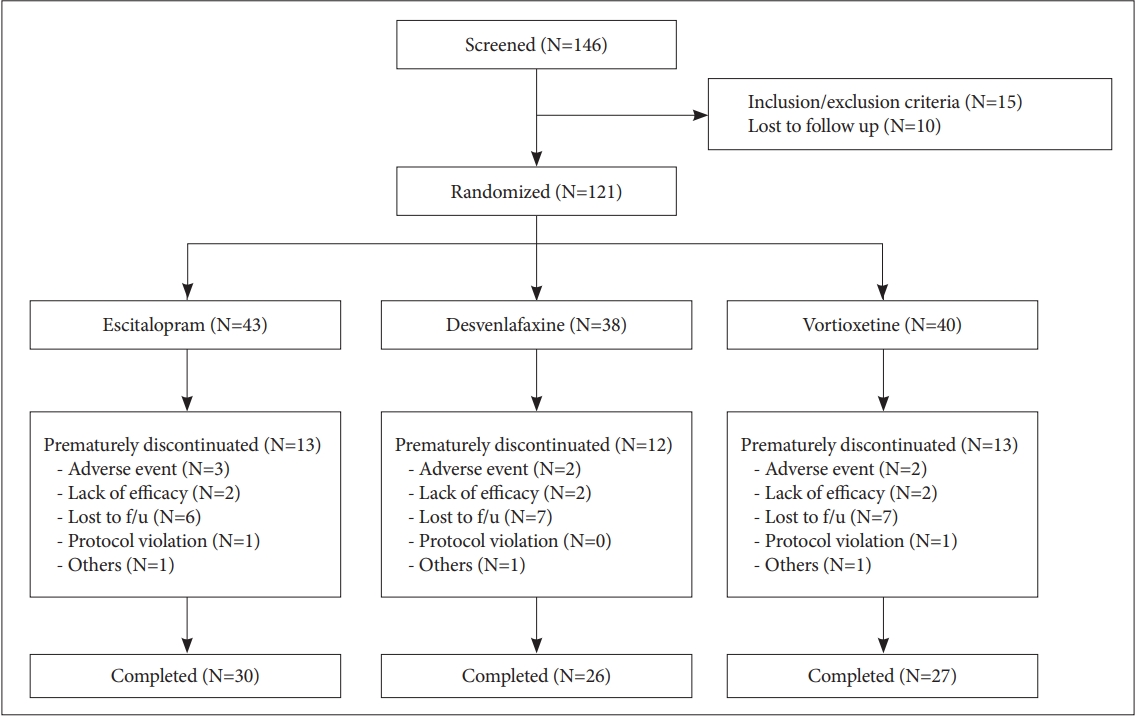
|
 |
- Search
| Psychiatry Investig > Volume 19(4); 2022 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
Supplementary Materials
Supplementary Table 1
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to privacy/ethical restriction but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Changsu Han, Sang Won Jeon, Hyonggin An. Data curation: Seung-Hoon Lee, Sang Won Jeon, Cheolmin Shin. Formal analysis: Seung-Hoon Lee, Sang Won Jeon, Hyonggin An. Funding acquisition: Changsu Han. Investigation: Seung-Hoon Lee, Sang Won Jeon, Cheolmin Shin, Chi-Un Pae, Changsu Han. Methodology: Changsu Han, Sang Won Jeon, Hyonggin An. Project administration: Changsu Han. Resources: Ashwin A. Patkar, Prakash S. Masand. Supervision: Changsu Han, Sang Won Jeon. Validation: Seung-Hoon Lee, Sang Won Jeon. Visualization: Seung-Hoon Lee, Sang Won Jeon. Writing—original draft: Seung-Hoon Lee, Sang Won Jeon. Writing—review & editing: Ashwin A. Patkar, Prakash S. Masand.
Funding Statement
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1405).
Figure 2.

Table 1.
Values are presented as number (%) or mean±standard deviation. HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; PDQ-D, Perceived Deficits Questionnaire-Depression; BC-CCI, British Columbia Cognitive Complaints Inventory; CGI-S, Clinical Global Impression-Severity; HAMA, Hamilton Anxiety Rating Scale; CUDOS, Clinically Useful Depression Outcome Scale; CUXOS, Clinically Useful Anxiety Outcome Scale; PHQ-15, Patient Health Questionnaire-15; GAF, Global Assessment of Functioning; WHOQOL-BREF, WHO Quality of Life Scale Abbreviated Version
Table 2.
| Variable change at week 6 | E (N=43) | D (N=38) | V (N=40) |
p |
Difference (p) |
||
|---|---|---|---|---|---|---|---|
| E vs. D vs. V | E vs. D | E vs. V | D vs. V | ||||
| HAMD | -10.0±1.0 | -13.9±1.6 | -8.9±1.2 | 0.025* | 0.106 | >0.999 | 0.028 |
| MADRS | -11.7±1.4 | -15.3±1.7 | -10.0±1.4 | 0.055 | 0.289 | >0.999 | 0.054 |
| PDQ-D | -11.7±2.2 | -14.4±3.2 | -11.5±2.4 | 0.701 | >0.999 | >0.999 | >0.999 |
| BC-CCI | -8.2±0.9 | -8.0±0.7 | -8.4±1.1 | 0.963 | >0.999 | >0.999 | >0.999 |
| CGI-S | -1.3±0.2 | -1.7±0.2 | -1.2±0.2 | 0.238 | 0.581 | >0.999 | 0.312 |
| HAMA | -13.0±1.8 | -16.1±1.8 | -10.2±1.4 | 0.059 | 0.606 | 0.654 | 0.054 |
| CUDOS | -14.1±2.0 | -16.9±2.1 | -11.7±2.1 | 0.244 | >0.999 | >0.999 | 0.282 |
| CUXOS | -13.4±3.4 | -20.3±3.4 | -12.9±2.1 | 0.058 | 0.073 | >0.999 | 0.069 |
| PHQ-15 | -3.9±0.7 | -4.8±1.2 | -2.8±0.7 | 0.244 | >0.999 | 0.974 | 0.289 |
| GAF | 9.0±1.4 | 9.2±1.9 | 8.8±1.5 | 0.365 | >0.999 | >0.999 | >0.999 |
| WHOQOL-BREF | 2.7±1.0 | 2.8±1.2 | 1.2±1.4 | 0.573 | >0.999 | >0.999 | >0.999 |
Values are presented as mean±standard error. Mean change was adjusted for age, sex, baseline HAMD score, baseline PDQ-D score, site, and benzodiazepine or zolpidem use at baseline.
E, escitalopram; D, desvenlafaxine; V, vortioxetine; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; PDQ-D, Perceived Deficits Questionnaire-Depression; BC-CCI, British Columbia Cognitive Complaints Inventory; CGI-S, Clinical Global Impression-Severity; HAMA, Hamilton Anxiety Rating Scale; CUDOS, Clinically Useful Depression Outcome Scale; CUXOS, Clinically Useful Anxiety Outcome Scale; PHQ-15, Patient Health Questionnaire-15; GAF, Global Assessment of Functioning; WHOQOL-BREF, WHO Quality of Life Scale Abbreviated Version
Table 3.
Table 4.
Values are presented as mean±standard error. Mean change was adjusted for age, sex, baseline HAMD score, baseline Perceived Deficits Questionnaire-Depression score, site, and benzodiazepine or zolpidem use at baseline. E, escitalopram; D, desvenlafaxine; V, vortioxetine; HAMD, Hamilton Depression Rating Scale
Table 5.
Values are presented as mean±standard error. Mean change was adjusted for age, sex, baseline Hamilton Depression Rating Scale score, baseline PDQ-D score, site, and benzodiazepine or zolpidem use at baseline. E, escitalopram; D, desvenlafaxine; V, vortioxetine; PDQ-D, Perceived Deficits Questionnaire-Depression
Table 6.
| Adverse event | Total (N=121) | Escitalopram (N=43) | Desvenlafaxine (N=38) | Vortioxetine (N=40) |
|---|---|---|---|---|
| Fatigue | 21 (17.4) | 8 (18.6) | 7 (18.4) | 6 (15.0)* |
| Anxiety/agitation | 19 (15.7) | 8 (18.6)* | 6 (15.8) | 5 (12.5) |
| Insomnia | 19 (15.7) | 7 (16.3) | 6 (15.8) | 6 (15.0) |
| Dry mouth | 19 (15.7) | 7 (16.3) | 6 (15.8) | 6 (15.0) |
| Somnolence | 16 (13.2) | 6 (14.0) | 6 (15.8) | 4 (10.0) |
| Headache | 14 (11.6) | 5 (11.6) | 5 (13.2) | 4 (10.0) |
| Constipation | 12 (9.9) | 5 (11.6) | 4 (10.5) | 3 (7.5) |
| Palpitation or tachycardia | 12 (9.9) | 3 (7.0) | 7 (18.4)* | 2 (5.0) |
| Memory impairment | 9 (7.4) | 3 (7.0) | 3 (7.9) | 3 (7.5) |
| Nausea/vomiting | 8 (6.6) | 2 (4.7) | 3 (7.9)* | 3 (7.5)* |
| Weight loss/decreased appetite | 6 (5.0) | 2 (4.7) | 2 (5.3) | 2 (5.0) |
| Increased sweating | 6 (5.0) | 2 (4.7) | 3 (7.9) | 1 (2.5) |
| Dizziness | 5 (4.1) | 2 (4.7) | 2 (5.3) | 1 (2.5) |
| Weight gain/increased appetite | 4 (3.3) | 1 (2.3) | 1 (2.6) | 2 (5.0) |
| Total | 170 | 61 | 61 | 48 |
REFERENCES