Acute Efficacy and Safety of Escitalopram Versus Desvenlafaxine and Vortioxetine in the Treatment of Depression With Cognitive Complaint: A Rater-Blinded Randomized Comparative Study
Article information
Abstract
Objective
This study aimed to compare the efficacy and safety of escitalopram, vortioxetine, and desvenlafaxine for acute treatment of major depressive disorder (MDD) with cognitive complaint (CC).
Methods
A total of 129 patients with MDD who also complained of CC were randomized evenly to either escitalopram, vortioxetine, or desvenlafaxine group and underwent a multi-center, six-week, rater-blinded, and head-to-head comparative trial. Differences in depressive symptoms following treatment were measured using the Hamilton Depression Rating Scale (HAMD) and the Montgomery-Åsberg Depression Rating Scale (MADRS). Subjective cognitive function and the presence of adverse events were assessed.
Results
The three antidepressant treatment groups did not show significant differences in the improvement of depressive symptoms as measured by HAMD and MADRS. Desvenlafaxine treatment was associated with a superior treatment response rate in depressive symptoms compared to vortioxetine or escitalopram treatment. However, no significant differences were found in the remission rate of depressive symptoms. The three antidepressant treatment groups did not show significant differences in the improvement of CC. Adverse profiles of each treatment group were tolerable, with no significant differences.
Conclusion
In acute antidepressant treatment for MDD with CC, escitalopram, vortioxetine, and desvenlafaxine presented similar efficacy in relief of depressive symptoms; however, desvenlafaxine was associated with a superior treatment. Further studies are needed to confirm these results by investigating the therapeutic efficacy and safety profile of long-term antidepressant treatment of MDD with CC (Clinical Trial Registry, http://cris.nih.go.kr/cris/en/: KCT0002173).
INTRODUCTION
Cognitive function in major depressive disorder (MDD) can be assessed by subjective or objective measures [1-3]. Cognitive complaint (CC) is a self-reported decline in cognitive function compared to a previous state [3,4]. CC is referred to with different nomenclatures in various studies. Subjective memory complaints, subjective cognitive decline, perceived forgetfulness, or cognitive concern constitute the operational definition for CC [3-6]. In some studies, CC is recognized as a possible identifier of future objective cognitive impairment and incipient dementia [7-10]. In other cases, CC is not correlated strongly with concurrent level of cognitive function as evaluated by objective measures [3,11]. Persons who complain of CC seem to have greater chance of future objective cognitive decline than do individuals who do not have CC. Also, some cases of CC accompanied by neurodegenerative features seem to be at higher risk of future objective cognitive dysfunction [12,13].
Despite ambiguous results on the relationship between objective cognitive impairment and CC, CC is common among individuals with MDD and has been reported to be associated with the presence and severity of depression in both community-dwelling and clinic-based samples [14]. In the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5), the diagnostic criteria for MDD include “diminished ability to think or concentrate either by subjective account or as observed by others.” [15] Thus, the MDD diagnostic criteria per se contain CC as a subjective cognitive symptom. A study describing the prevalence of patient-reported symptoms in MDD identified “trouble in concentration,” which is a subjective cognitive symptom of MDD, as the second most frequently reported symptom in depressive episodes, which was reported in 73.5% of the patients [16,17]. In a recent study, CC was associated more highly with the severity of depression and general functioning than was objective cognitive impairment [18]. CC in MDD patients is correlated more closely with not only depressive symptoms, but also psychosocial functioning compared to objective cognitive impairment [19-23]. Considering recent evidence suggesting a closer relationship between CC and depression than between objective cognition and depression, CC might have a greater impact on depression than objective cognitive decline.
The currently available treatment guidelines for MDD typically suggest antidepressant monotherapy in major depressive episodes [24-26]. A series of industry-sponsored, double-blind, and randomized placebo-controlled clinical trials have shown the efficacy of the antidepressant vortioxetine on objective cognitive function, subjective CC, and depressive symptoms in MDD [27,28]. In the same trial, duloxetine displayed superior efficacy on subjective CC and depressive symptoms but not on objective cognitive function improvement in MDD patients [27]. These results, along with evidence from a meta-analysis of the efficacy of antidepressants on the objective cognitive function of MDD patients, led to the recommendation of vortioxetine to MDD patients with cognitive impairment in the Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder [26,29].
Reddy et al. [30]. examined the effects of desvenlafaxine at a dose of 50 mg/day on objective cognitive function of MDD patients. In a 12-week, randomized, double-blind, and placebocontrolled trial, desvenlafaxine displayed significant improvement on working memory. However, subjective CC was not investigated in the study [30]. In a study that compared the effects of the selective serotonin reuptake inhibitor (SSRI) escitalopram (n=36) and the serotonin-norepinephrine reuptake inhibitor (SNRI) duloxetine in treating objective cognitive deficit for 24 weeks, both antidepressant treatments improved objective cognitive function [31]. The improvement was notable in episodic memory and to a lesser extent in working memory, mental processing speed, and motor performance. However, subjective CC was not examined in the study [31].
Considering the effects of subjective CC on depressive disorders with respect to the poor clinical course and low levels of psychosocial functioning, an improved strategy for management of MDD with accompanying CC is needed. Despite the evidence on vortioxetine use in MDD patients with CC, studies to evaluate the efficacy of antidepressants other than vortioxetine and duloxetine in patients with both MDD and CC are scarce. Thus, it is imperative to compare the treatment outcomes of pharmacological treatments on MDD with accompanying CC.
The primary aim of this study was to compare the efficacy of three antidepressants (escitalopram, vortioxetine, and desvenlafaxine) in ameliorating depressive symptoms. The secondary aim of the study was to compare the outcomes of the three antidepressant treatments for improvement of CC, anxiety symptoms, quality of life (QoL), trajectory of functional status, and adverse effects. The intention of this study was to provide a resource to aid in choosing the optimal antidepressant treatment in MDD accompanied by CC.
METHODS
Participants
Subjects aged 19 to 65 years were drawn from outpatients who fulfilled the criteria for MDD without psychotic features according to DSM-5. Inclusion criteria were 1) subjects who exhibited a baseline score ≥14 on the 17-item Hamilton Depression Rating Scale (HAMD), 2) subjects who had subjective CC at baseline, and 3) subjects who did not receive adequate antidepressant treatment, defined as ≥4 consecutive weeks of treatment at the recommended dosage for the particular antidepressant, prior to participation of the current study.
Exclusion criteria were current or past comorbid diagnosis of schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, a psychotic disorder not otherwise specified, mood-congruent or mood-incongruent psychotic features, bipolar disorder, alcohol or substance-use disorder, organic mental disorder including dementia, eating disorder, or obsessive compulsive disorder; presence of a seizure disorder or comorbid serious medical illness including hyperor hypothyroidism; previous treatment with electroconvulsive therapy; currently pregnant/lactating women; and risk of suicide. Subjects with an unclear history of antidepressant treatment prior to the study entry were excluded.
All patients provided medical and psychiatric history, and physical and routine laboratory examinations were carried out at the onset of the study. After a complete description of the study, written informed consent was obtained from all patients. This study was conducted in accordance with the code of ethics of the World Medical Association (i.e., Declaration of Helsinki). The current study was preregistered with the Clinical Research Information Service (CRIS) of the Korea Disease Control and Prevention Agency (Clinical Trial Registry, http://cris.nih.go.kr/cris/en/: KCT0002173).
All participants provided written consent, and the protocol was approved by the Institutional Review Board of the Kangbuk Samsung Hospital (IRB No. KBSMC 2018-04-004).
Treatment protocol
This was a 6-week, prospective, randomized, rater-blinded, and active-controlled trial conducted from September 2016 through December 2018 at five university hospitals across South Korea. Eligible subjects were randomized in a 1:1:1 ratio to receive one of three treatment arms: escitalopram, desvenlafaxine, or vortioxetine. Drug dosages and titration schedules were based on the recommendations of the prescribing information for each product and according to the judgment of the clinicians involved in the study (escitalopram [10–20 mg/day], desvenlafaxine [50–200 mg/day], or vortioxetine [10–20 mg/ day]). No other psychotropic drugs were allowed during the study period except benzodiazepines (up to 4 mg/day of lorazepam or equivalent) and hypnotics (up to 10 mg/day of zolpidem or equivalent).
Assessments
Study patients were assessed at baseline and 2, 4, and 6 weeks. The primary efficacy variables were change in the 17- item HAMD and change in the Montgomery-Åsberg Depression Rating Scale (MADRS). The secondary efficacy measures were changes in the Perceived Deficits Questionnaire-Depression (PDQ-D) and British Columbia Cognitive Complaints Inventory (BC-CCI). Response was defined as a HAMD/ MADRS score improvement greater than 50% at the endpoint compared with baseline, and remission was defined as an HAMD score of 7 or less and a total MADRS score of 12 or less at the endpoint. Other instruments used were the Clinical Global Impression-Severity (CGI-S), Hamilton Anxiety Rating Scale (HAMA), Clinically Useful Depression Outcome Scale (CUDOS), Clinically Useful Anxiety Outcome Scale (CUXOS), Patient Health Questionnaire-15 (PHQ-15), Global Assessment of Functioning (GAF), and WHO Quality of Life Scale Abbreviated Version (WHOQOL-BREF). All assessors completed the same training module and were blinded to the patients’ conditions and prescribed medications.
Safety was assessed via adverse events, vital signs, weight, and physical examination findings at each visit. Adverse events during the study period were recorded using the Systematic Assessment for Treatment Emergent Events-Specific Inquiry and were evaluated for severity and causal relationship to the study drug.
Perceived Deficits Questionnaire-Depression
The PDQ-D is a brief, self-reported questionnaire for evaluation of subjective cognitive symptoms in depressive patients. The PDQ-D consists of 20 items that represent four domains of cognitive function: attention/concentration, retrospective memory, prospective memory, and organization/planning. Each respondent rates the frequency of each complaint on a 5-point Likert scale anchored from never [0] to almost always [4] in a self-report form; each domain is scored out of 20, and a higher score indicates more severe cognitive dysfunction [32]. The Korean version of the PDQ-D has been validated [2].
British Columbia Cognitive Complaints Inventory
The BC-CCI is a self-rated, rapid screening tool that assesses perceived cognitive difficulties specifically in patients with depression. The scale consists of 6 items assessing perceived problems with concentration, memory, thought expression, word choice, slow thinking, and difficulty solving problems in the past 7 days. Scores on each item (ranging from 0, not at all, to 3, very much) are summed to yield a total score ranging from 0 to 18; scores of 0–4 are normal [33].
Statistical analysis
Subjects who were randomized and received one or more doses of the study drug and had one or more post-baseline values for the primary and secondary efficacy assessments were included in the analysis set. All outcome measures are presented as differences between the three groups of escitalopram vs. desvenlafaxine vs. vortioxetine. We compared baseline demographics and clinical characteristics among the groups using analysis of variance (ANOVA), the chi-square test, or Fisher’s exact test.
The primary endpoint (mean change in the total scores of HAMD and MADRS relative to baseline scores) was analyzed by a mixed model for repeated measures analysis of covariance (ANCOVA), with the treatment group as the betweensubject factor. Age, sex, baseline HAMD score, baseline PDQ-D score, regional center variability, benzodiazepine or zolpidem use at baseline, and other variables that were significantly different at baseline comparison were considered as the covariates. Secondary endpoints (PDQ-D and BC-CCI) and other variables (CGI-S, HAMA, CUDOS, CUXOS, PHQ-15, GAF, and WHOQOL-BREF) were analyzed in a similar manner to the primary endpoint. Response and remission rates were analyzed by multivariate logistic regression, with the same method as in the ANCOVA described above. Missing values were inputted using the last observation by carried forward approach. Additionally, the change in depressive symptom and CC in individual study groups were evaluated with paired t-test of mean measure score (HAMD, MADRS, PDQ-D, and BC-CCI) between baseline and end-point.
Serious adverse events (AEs) were recorded from the date of informed consent to the last follow-up contact, and other AEs were documented from the beginning of drug administration to the end of the follow-up period. In the present analysis, cases with items that were associated with more than 5% of subjects were considered significant drug-related AEs. AEs leading to discontinuation of the study drug or withdrawal from the study were documented.
Statistical significance was set at p<0.05 (two-tailed) for all tests. All statistical analyses were conducted using the Statistical Analysis System (SAS, version 9.1; SAS Institute, Inc., Cary, NC, USA) software package.
RESULTS
Baseline characteristics
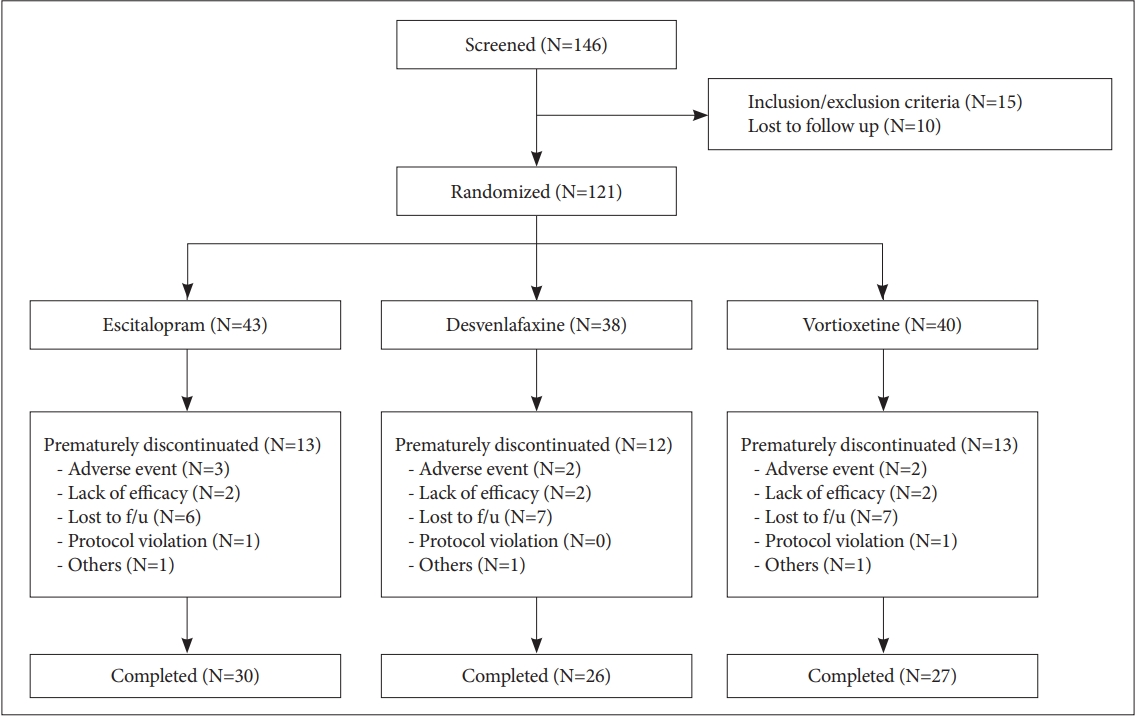
Of a total of 146 patients screened in the present study (Figure 1), 121 met inclusion criteria and were randomized to receive the study drugs (escitalopram, n=43; desvenlafaxine, n=38; vortioxetine, n=40). Baseline sociodemographic and clinical characteristics of the subjects are summarized in Table 1. The mean HAMD total score of the subjects at baseline was 23.4, and the mean MADRS score was 29.3, indicating that the subjects experienced moderately severe events. The mean scores for PDQ-D and BC-CCI were 42.8 and 11.6, respectively, indicating moderate-to-severe CC.
When comparing baseline characteristics of the three groups, there were no significant differences in socio-demographic or clinical characteristics (Table 1). There was no significant difference in the total scores of HAMD (p=0.207), MADRS (p= 0.355), PDQ-D (p=0.434), or BC-CCI (p=0.985) at baseline. Further, the total scores of CGI-S, HAMA, CUDOS, CUXOS, PHQ-15, GAF, and WHOQOL-BREF at baseline were not significantly different among the groups.
The mean doses of the antidepressant during the study period were as follows (Supplementary Table 1 in the online-only Data Supplement): 6.0±1.9 mg/day at weeks 0–2, 11.2±3.3 mg/day at weeks 2–4, 14.3±4.3 mg/day at weeks 4–6, and 15.3±5.3 mg/day at week 6 in the escitalopram group; 65.2±21.2 mg/day at weeks 0–2, 98.3±35.1 mg/day at weeks 2–4, 125.5±50.7 mg/day at weeks 4–6, and 135.2±60.0 mg/day at week 6 in the desvenlafaxine group; 6.0±2.0 mg/day at weeks 0–2, 12.2± 3.7 mg/day at weeks 2–4, 15.7±5.3 mg/day at weeks 4–6, and 16.5±5.7 mg/day at week 6 in the vortioxetine group. The mean lorazepam-equivalent doses and amounts of zolpidem used during the study period were not significantly different among the groups at visitation points (Supplementary Table 1 in the online-only Data Supplement).
Analyses of efficacy
In the primary efficacy analysis, there were statistically significant differences between escitalopram, desvenlafaxine, and vortioxetine in baseline-to-endpoint improvement in HAMD total score (overall p=0.025) after adjusting for potential confounding variables, as shown in Table 2. However, these differences between escitalopram vs. desvenlafaxine (p=0.106), escitalopram vs. vortioxetine (p>0.999), and desvenlafaxine vs. vortioxetine (p=0.028) did not reach significance at week 6 after Bonferroni correction for multiple comparisons. There were no significant differences between the three groups in MADRS change from baseline to week 6 (p=0.055).
In the secondary efficacy analysis, there was no difference between the three groups in PDQ-D (p=0.701) or BC-CCI (p= 0.963) change from baseline to week 6. Other variables (CGI [p=0.238], HAMA [p=0.059], CUDOS [p=0.244], CUXOS [p=0.058], PHQ-15 [p=0.244], GAF [p=0.365], and WHOQOL- BREF [p=0.573]) did not show significant differences among the three groups. All treatment arm displayedan improvement in depressive symptoms or CC, with no differences among treatments at any time point throughout the treatment (Figure 2 and Supplementary Table 2 in the online-only Data Supplement).

Mean changes between follow-up visits. *statistically significant change in the mean score between baseline and end-point (p<0.05, paired t-test). HAMA, Hamilton Anxiety Rating Scale; PDQD, Perceived Deficits Questionnaire-Depression.
The change in the mean score of HAMD, MADRS, PDQ-D, and BC-CCI between the baseline and the endpoint in each group were assessed via paired t-test. All three antidepressants displayed statistically significant change in the mean score of HAMD, MADRS, PDQ-D, and BC-CCI from baseline to end-point (HAMD [escitalopram: t=9.513, p<0.001; desvenlafaxine: t=8.477, p<0.001; vortioxetine: t=7.112, p<0.001]) (MADRS [escitalopram: t=8.200, p<0.001; desvenlafaxine: t=9.249, p<0.001; vortioxetine: t=6.934, p<0.001]) (PDQ-D [escitalopram: t=8.285, p<0.001; desvenlafaxine: t=4.437, p< 0.001; vortioxetine: t=4.768, p<0.001]) (BC-CCI [escitalopram: t=3.064, p=0.005; desvenlafaxine: t=3.655, p=0.001; vortioxetine: t=2.280, p=0.031]) (Figure 2).
The unadjusted MADRS response rate was significantly different among the three groups (χ2=11.038, degrees of freedom [df]=2, p=0.004). The MADRS response at week 6 was achieved in 25.6% (n=11), 44.7% (n=17), and 17.5% (n=7) of the subjects in the escitalopram, desvenlafaxine, and vortioxetine groups, respectively. There was no significant difference among the three groups in unadjusted HAMD response rate (χ2=4.910, df=2, p=0.086), HAMD remission rate (χ2=1.438, df=2, p=0.487), or MADRS remission rate (χ2=0.067, df=2, p= 0.823). After adjusting for potential confounding variables, the desvenlafaxine group showed a significantly higher response rate according to HAMD (odds ratio [OR]=1.71, 95% confidence interval [CI]=1.07–3.91) and MADRS (OR=1.63, 95% CI=1.27–2.42) compared to the vortioxetine group (Table 3). Compared to the escitalopram group, the desvenlafaxine group showed a significantly higher response rate for MADRS (OR= 3.97, 95% CI=1.25–12.63).
The remission rate of each antidepressants based on HAMD score were 16.3% (n=7), 23.7% (n=9) and 17.5% (n=7) for escitalopram, desvenlafaxine, and vortioxetine, respectively. The remission rate of individual antidepressant based on MADRS score were 18.6% (n=8), 18.4% (n=7), 17.5% (n=7) for escitalopram, desvenlafaxine, and vortioxetine, respectively. There were no significant differences among the three groups in ad- justed HAMD and MADRS remission rates (Table 3).
Analysis of individual HAMD items reveled no significant differences among the three treatments (Table 4). There were no significant differences between the three groups for individual PDQ-D factor scores (Table 5).
Analyses of discontinuations and adverse events
Thirty-eight subjects prematurely discontinued treatment, with the most common reasons of loss to follow-up (n=20), AEs (n=7), insufficient treatment response (n=6), or protocol violation (n=2). The drop-out rate was 30.2% (n=13) in the escitalopram group, 31.6% (n=12) in the desvenlafaxine group, and 32.5% (n=13) in the vortioxetine group and was not significantly different among the groups (χ2=1.656, df=2, p=0.542). No statistically significant differences were evident among the treatment groups for any reasons for discontinuation.
A total of 47 patients (38.8%) reported 170 cases of AEs. The percentage of subjects who reported at least one AE during the study period was 39.2%, 43.2%, and 35.1% in the escitalopram, desvenlafaxine, and vortioxetine groups, respectively (χ2=0.815, df=2, p=0.213). The most frequently reported AEs—which were reported in at least 5% of the subjects in any of the treatment groups—were fatigue, anxiety/agitation, insomnia, dry mouth, somnolence, headache, constipation, palpitations/ tachycardia, memory impairment, nausea/vomiting, weight loss, increased sweating, dizziness, and weight gain (Table 6).
Seven subjects had AEs leading to study discontinuation: 3 (7.0%), 2 (5.3%), and 2 (5.0%) subjects in the escitalopram, desvenlafaxine, and vortioxetine groups, respectively. AEs leading to treatment discontinuation were fatigue (n=2) and agitation (n=1) in the escitalopram group, palpitations (n=1) and nausea (n=1) in the desvenlafaxine group, and fatigue (n=1) and vomiting (n=1) in the vortioxetine group.
DISCUSSION
The current study evaluated the efficacy (i.e., reduction in depressive symptoms and subjective cognitive function) and safety of six-week acute antidepressant treatment in MDD patients who complained of CC by directly comparing antidepressant pharmacological treatments (escitalopram, vortioxetine, and desvenlafaxine). There was no significant difference in the degree of depressive symptom reduction as measured by the symptom scales between the pharmacological treat- ments. Nonetheless, desvenlafaxine treatment was associated with a superior treatment response rate for depressive symptoms compared to treatment with vortioxetine or escitalopram. There was no significant difference in the remission rate of depression between the medications. The degree of CC improvement showed no significant difference between the pharmacological treatments. Furthermore, despite the improvement, substantial CC remained even after 6 weeks of treatment. The current study also compared anxiety symptoms, functional status, and QoL among the treatment methods. No significant differences were observed regarding anxiety, functional status, or QoL. The AEs and safety of treatment were evaluated among treatment groups and showed no differences in type, frequency, or distribution of AEs or the drop-out rate.
In Sequenced Treatment Alternatives to Relieve Depression (STAR-D) study, a remission rate of 28% as assessed by HAMD was achieved with a mean citalopram dose 41.8 mg prescribed by physician for up to 14 weeks [34]. Escitalopram displayed the remission rate of 27.2% among patients with moderate to severe severity MDD, in a pooled analysis of 4 clinical trials where the average dosage of escitalopram was 14.2± 4.9 mg [35]. In a 8 week double-blind randomized, placebo-controlled study assessing the efficacy of desvenlafaxine on MDD, HAMD remission rate were 23% and 17% in 50 mg/day and 10 mg/day desvenlafaxine treatment group [36]. In other 8 week, double-blind, placebo-controlled study evaluated the efficacy of desvenlafaxine on MDD, the remission rate assessed with HAMD were 32% and 28% in desvenlafaxine 400 mg and 200 mg group, respectively [37]. MADRS remission rate of vortioxetine in a 8 week randomized, double-blind, placebo-controlled study on MDD with CC were 29.5% and 38.2% in treatment group with 10 mg/day and 20 mg/day vortioxetine, repectively [38]. Considering the result from previous studies and the duration of current study, current study displayed comparable remission rate with other clinical trials in which sought for the acute efficacy of antidepressants that are used in current study.
Escitalopram, desvenlafaxine, and vortioxetine were all found to have effective treatment responses and tolerable safety in a network meta-analysis [39]. The odds ratio of the drop-out rate compared with that of a placebo-controlled group was estimated in this network meta-analysis, indicating that escitalopram has a slightly superior acceptability compared with desvenlafaxine or vortioxetine. Moreover, in an indirect comparison of head-to-head trials, escitalopram, desvenlafaxine, and vortioxetine showed no significant difference in treatment response or drop-out rate [39]. The results of a network metaanalysis of acute phase antidepressant treatment of MDD agreed with the results of the current study, with the exceptions that desvenlafaxine presented a superior treatment response rate compared to escitalopram and vortioxetine, and no differences in safety profiles among the three antidepressants used in the current study were seen. In the network meta-analysis by Cipriani et al. [39], cognitive function was not investigated, and cognitive dysfunction was not used as a study outcome. Based on the results of the current study, acute-phase treatment with desvenlafaxine may be more effective at ameliorating depressive symptoms for MDD patients with accompanying CC compared to other medications.
In a randomized controlled trial examining the effects of antidepressants on MDD with accompanying CC, vortioxetine demonstrated efficacy in treating depressive symptoms, subjective CC, and objective cognitive function, while duloxetine displayed significant improvement only on depressive symptoms and subjective CC [27]. To our best knowledge, studies evaluating the efficacy of antidepressants other than vortioxetine or duloxetine on MDD with accompanying CC are scarce. The current study also found improvement of depressive symptoms by vortioxetine treatment, consistent with a previous study by Mahableshwarkar et al. [27]. In addition to the consistant result with previous studies, the current study also found that escitalopram and desvenlafaxine displayed comparable efficacy in acute treatment of MDD patients with accompanying CC.
The existing literature contains different definitions of CC. Although some studies state that patients with CC must not manifest a reduction in objective cognition, others define CC as a subjective complaint of cognitive deficit regardless of the presence of objective cognitive impairment [3,40]. Despite the differences in the definition of CC in the reported literature, most studies concordantly state that CC should be defined as a subjective patient complaint of decline in at least one of the domains of cognitive function [40]. All participants of the current study complained of CC; however, objective cognitive impairment was not investigated using objective measures.
Even for MDD patients complaining of CC without a decline in objective cognitive function, CC can be associated with future objective cognitive dysfunction [7-10]. Thus, despite lack of investigation of objective cognitive function in the current study, patients complaining of CC can present future objective cognitive problems compared to MDD patients who do not complain of CC. Hence, CC should be evaluated in MDD patients. The current evidence of treatment responses in MDD patients with accompanying CC can help guide treatment options. However, to extend these results to a wide variety of CCs, further studies investigating the effects of alleviating CC and depressive symptoms through antidepressant treatments on objective cognitive impairment are needed.
Previous studies have shown the potential of antidepressants for improving not only mood symptoms, but also cognitive function. In a meta-analysis of 9 randomized controlled trials examining antidepressant treatments for MDD patients in which cognitive function was evaluated, antidepressants were associated with positive effects on psychomotor speed and delayed recall [29]. However, no significant improvement in cognitive control or executive function was identified in that study. Escitalopram treatment in stroke patients has shown improvement in global cognitive functioning, specifically in verbal and visual memory functions, compared to individuals on placebo or problem-solving therapy [41]. In an open-label study, depressed elderly patients had markedly reduced memory performance compared to healthy controls, while treatment with escitalopram significantly improved affective and cognitive symptoms of depressed patients [42].
In addition to the evidence supporting the efficacy of vortioxetine on MDD with cognitive impairment from an industry- sponsored randomized control study and CANMAT guidelines, the European Medicines Agency updated the clinical efficacy of vortioxetine in reference to its effect on cognitive function [26-28,43]. In a sub-study of a randomized, double-blind, placebo-controlled study, desvenlafaxine treatment showed significant improvements on both the quality and speed of working memory [30]. In a placebo-controlled study of desvenlafaxine, an eight-week treatment led to a significant improvement in depressive symptoms, functional outcomes, and perceived cognitive functioning [44]. In summary, all the medications used in the current study have the potential to improve cognitive function in MDD as indicated by prior studies.
The drugs used in the current study have distinct mechanisms of action, which can have different effects on MDD accompanied by CC. Various hypotheses on cognitive dysfunction of mood disorders have been suggested. The dysregulation of neurotransmitters, serotonin and noradrenaline in particular, that is associated with depression is thought to be a causal mechanism in cognitive impairment associated with MDD [30,45,46]. Escitalopram is a SSRI, and pure serotonin reuptake transporter (SERT) inhibition is likely to explain almost all of its pharmacological actions [47]. Vortioxetine not only inhibits serotonin (5-HT) reuptake mediated by SERT, but also modulates other 5-HT receptor activities, including antagonistic action toward ligand-dependent ion channels, such as the 5-HT3 receptor [46]. In an animal model of depression and cognitive dysfunction, ondansetron, a 5-HT3 receptor antagonist, rescued deficits in spatial and fear-related memory, implying a role of 5-HT3 receptor in cognitive function of depressed patients [46].
Desvenlafaxine is an active metabolite of the SNRI venlafaxine. Desvenlafaxine displays greater noradrenaline transporter (NET) inhibition compared to venlafaxine, which inhibits SERT. Noradrenergic (NE) neurons project to the limbic system, the functions of which are associated with emotion and cognition [48,49]. Stahl [50] suggested that, in neuroanatomical terms, emotional functional centers in the brain receive input from both NE and 5-HT neuronal projections, while cognitive functional centers receive direct projections from NE, dopaminergic, and histaminergic but not 5-HT neurons. Since desvenlafaxine presented a significant favorable treatment response in ameliorating depressive symptoms and has direct effects on NE neurotransmission, NE transmission might have contributed to the better treatment responses in improving depressive symptoms. However, the causal relationship between the antidepressant’s neurobiological mechanism of action and clinical treatment outcome requires further study.
Benzodiazepine effects on cognitive function can dampen the treatment effects on CC. The acute effects of benzodiazepine use include sedation, drowsiness, mental slowing, and anterograde amnesia [51-53]. In a systematic review of all randomized, double-blind, placebo-controlled studies with detailed neurocognitive measures prior to and after administration of oral benzodiazepines, its use has been shown to provoke amnestic and non-amnestic impairments [51,54]. Thus, considering the use of benzodiazepine among the current study subjects, there is a possibility that the cognitive improvement effect of antidepressant treatment was hindered by the adverse effect of benzodiazepine on cognition. Thus, the antidepressants may have had greater improvement on CC in the absence of benzodiazepine use.
The current study has several limitations. First, the diagnoses of MDD were not based on structured clinical interviews as indicated in the DSM-5. Second, the clinical trial was rater- blinded but not double-blinded, and it was an open-label study. Further, the dosage of the drugs was not fixed but depended on the judgment of a clinician. Additionally, the relatively high drop-out rate in the current study can limit the interpretation of the results. Up to 20% of patients typically drop-out during a clinical trial, while the current study had a drop-out rate in the range of 30.2% to 32.5% depending on the medication treatment group [55]. Lack of an objective assessment for cognitive function limits interpretations of the study results for MDD with accompanying CC. However, indirect evidence from prior longitudinal studies indicating an association between CC and future objective cognitive dysfunction suggests the potential to adapt the current study results to cases of objective cognitive impairment [7-10]. Since the current study assessed only acute treatment outcomes following a 6-week treatment, outcomes of longer treatment should be explored in future studies, especially when considering that cognitive changes after antidepressant treatment have been reported in a previous study with a longer treatment period [56].
In conclusion, acute antidepressant treatment of MDD ac companied by CC showed that escitalopram, vortioxetine, and desvenlafaxine presented similar treatment outcomes for depressive symptoms, with the exception that desvenlafaxine had a superior response rate with respect to depressive symptoms. Subjective cognitive function, anxiety symptoms, QoL measure, and psychosocial function were not significantly different following acute treatment of MDD with accompanying CC. Furthermore, the safety profiles of the three medications did not differ. Based on this study, desvenlafaxine is the optimal treatment option for the treatment of MDD with CC. However, further studies are required to confirm this result by investigating the therapeutic efficacy and safety profile of longterm antidepressant treatment of MDD with CC and by exploring the underlying biological mechanisms of the different medications in MDD with CC.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2021.0368.
Doses of antidepressants and benzodiazepine and number of zolpidem uses at each visit
Mean changes between visits
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to privacy/ethical restriction but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Changsu Han, Sang Won Jeon, Hyonggin An. Data curation: Seung-Hoon Lee, Sang Won Jeon, Cheolmin Shin. Formal analysis: Seung-Hoon Lee, Sang Won Jeon, Hyonggin An. Funding acquisition: Changsu Han. Investigation: Seung-Hoon Lee, Sang Won Jeon, Cheolmin Shin, Chi-Un Pae, Changsu Han. Methodology: Changsu Han, Sang Won Jeon, Hyonggin An. Project administration: Changsu Han. Resources: Ashwin A. Patkar, Prakash S. Masand. Supervision: Changsu Han, Sang Won Jeon. Validation: Seung-Hoon Lee, Sang Won Jeon. Visualization: Seung-Hoon Lee, Sang Won Jeon. Writing—original draft: Seung-Hoon Lee, Sang Won Jeon. Writing—review & editing: Ashwin A. Patkar, Prakash S. Masand.
Funding Statement
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC15C1405).