 |
 |
- Search
| Psychiatry Investig > Volume 17(2); 2020 > Article |
|
Abstract
Objective
It is well established that the cortico-striato-thalamo-cortical (CSTC) circuit is implicated in the pathophysiology of obsessive- compulsive disorder (OCD). However, reports on corticostriatal functional connectivity (FC) in OCD have been inconsistent due to the structural and functional heterogeneity of the striatum. Therefore, in the present study, we investigated corticostriatal FC using a fine 12-seed striatal parcellation to overcome this heterogeneity and discover the neural correlates of symptoms in OCD patients.
Methods
We recruited 23 OCD patients and 23 healthy controls (HCs). Whole-brain FC based on striatal seeds was examined using resting-state functional magnetic resonance imaging data and compared across OCD patients and HCs. We conducted correlation analysis between FCs of striatal subregions with significant group differences and symptom severity scores on the Yale-Brown Obsessive Compulsive Scale (Y-BOCS), Hamilton Rating Scale for Depression, and Hamilton Rating Scale for Anxiety (HAM-A).
Results
Compared to HCs, patients demonstrated increased FC of the dorsal caudal putamen and ventral rostral putamen (VRP) with several cortical regions, such as the intracalcarine cortex, inferior frontal gyrus, supramarginal/angular gyrus (SMG/AG), and postcentral gyrus (PCG). Furthermore, FC between the VRP and SMG/AG and between the VRP and PCG was negatively correlated with scores on the Y-BOCS compulsive subscale and the HAM-A, respectively.
Conclusion
These findings suggest that striatal subregions have strengthened FC with extensive cortical regions, which may reflect neural correlates of compulsive and anxious symptoms in OCD patients. These results contribute to an improved understanding of OCD pathophysiology by complementing the current evidence regarding striatal FC.
Obsessive-compulsive disorder (OCD) is a relatively frequent mental disorder, with a lifetime prevalence of approximately 2-3% [1]. Patients with OCD are characterized by recurrent unwanted thoughts, images, or impulses and repetitive behaviors to relieve anxiety, often accompanied by obsessions [2,3]. OCD causes serious difficulties in daily life; thus, numerous studies have been carried out to clarify its pathophysiology [4]. Through such research, the cortico-striatothalamo-cortical (CSTC) circuit has emerged as a prevailing model of the neural underpinnings of OCD [3,5-7]. Based on convergent findings from neuroimaging studies, the hyperactive CSTC circuit in patients with OCD has been suggested as a cause of recurrent and exaggerated concerns about danger, order, hygiene, or harm and subsequently drives patients to respond with compulsive behaviors aimed at neutralizing the anxiety [8].
The striatum is one of the most important components of the CSTC circuit. This structure receives and coordinates multiple inputs from the cortical areas and delivers them to the thalamus [9-11]. Through this relay function, the striatum is involved in extensive cognitive and executive functions such as verbal and spatial working memory, response inhibition, task switching, and motor planning [9,12-14]. As the roles of the striatum in OCD have been emphasized, much has been revealed about striatal structures in OCD patients. Previous studies have reported mixed results regarding volumetric changes of the striatum. Scarone et al. [15] and Pujol et al. [16] observed that the right caudate and bilateral ventral putamen had greater volume in patients with OCD than in healthy controls (HC), while Kang et al. [17] and Riffkin et al. [18] did not find any significant changes in striatal volume in OCD patients. White matter structure has also been assessed with diffusion tensor imaging (DTI). Fan et al. [19] demonstrated reduced fractional anisotropy (FA) and increased radial diffusivity (RD) of the white matter around the left striatum in OCD. That team also confirmed that the RD value was negatively correlated with scores on the compulsive subscale of the Yale-Brown Obsessive Compulsive Scale (Y-BOCS).
Following studies on structural change, functional aspects have also been identified using resting-state functional magnetic resonance imaging (rs-fMRI). A number of studies have examined striatal functional connectivity (FC) in patients with OCD using the striatum as a seed region of interest (ROI). Many such studies have reported stronger FC between the ventral caudate and the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), dorsomedial prefrontal cortex (DMPFC), and dorsolateral prefrontal cortex (DLPFC) in OCD patients than in HCs [20-23]. In addition, some such studies have demonstrated weaker FC between the dorsal caudate and the DLPFC and ventrolateral prefrontal cortex (VLPFC) in OCD patients than in HCs [22,23]. Among these significant alterations, the FC between the ventral caudate and OFC was positively correlated with patients’ YBOCS scores [20,22]. However, numerous inconsistent results have also been reported. Harrison et al. [22] discovered that OCD patients showed weakened FC between the ventral putamen and inferior frontal cortex and between the ventral caudate and superior temporal cortex compared to HCs [20,22]. Chen et al. [24] observed strengthened FC between the left caudate and several cortical areas including the superior/middle temporal gyrus, middle/inferior occipital gyrus, and postcentral gyrus in patients with OCD. More recently, Vaghi et al. [23] found that the FC between the dorsal caudate and the parietal cortex is weaker in OCD patients than in HCs.
The results of studies on striatal FC in patients with OCD are still inconsistent. We speculated that the different striatal seeds used in each study might have contributed to this issue. The striatum is a heterogeneous structure that consists of several subregions, each of which is involved in different neurocognitive functions in connections with corresponding cortical areas [2,9,10]. Therefore, it is important to divide the striatum into functionally and structurally meaningful subregions when examining corticostriatal FC. Di Martino et al. [9] parcellated the striatum into 12 subdivisions based on extensive neuroimaging studies, and this parcellation has been widely used to investigate corticostriatal dysfunctions in psychiatric disorders [9,25-27]. In OCD, Posner et al. [28] applied these 12 striatal seeds for the first time to assess the corticostriatal FC alterations. That team observed that patients with OCD exhibited weakened FC between the ventral striatum and the ACC and OFC and between the dorsal caudal putamen and the supplementary motor area in comparison with HCs. On the other hand, the FC between the dorsal caudate and the prefrontal cortex (PFC) and inferior parietal cortex were stronger in OCD patients than in HCs. Furthermore, the investigators identified that the FC between the inferior ventral striatum and the ACC was negatively correlated with YBOCS scores. Notably, the directions of most findings in that study were the opposite of previous observations. Although the researchers explained this discrepancy with their unmedicated patients and the use of different striatal seeds, further evidence is needed to validate their results. In addition, a multiple comparison problem needs to be addressed in FC analysis using multiple striatal seed regions, despite not having been considered in most previous striatal FC studies, including that of Posner et al. [28].
In the present study, we aimed to identify corticostriatal dysfunction and its role as a neural correlate of psychiatric symptoms in patients with OCD using a fine 12-seed striatal parcellation [9]. We first intended to investigate whether the findings of Posner et al. [28] would be replicated in our patients even after a Bonferroni correction for multiple comparisons. Additionally, we expected to discover some additional cortical areas with altered FC to striatal subregions, which might not have been found in earlier studies that did not use finely parcellated striatal subregions as seed ROIs. Finally, we hypothesized that altered striatal FC would be correlated with symptomatic severity in OCD patients, serving as a neural correlate of psychiatric symptoms of OCD.
A total of 23 OCD patients and 23 HCs who were matched for age, gender, and handedness participated in this study. All patients with OCD were recruited from the outpatient clinic at Seoul National University Hospital (SNUH) and fulfilled the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for OCD. The severity of clinical symptoms was evaluated by the Y-BOCS, Hamilton Rating Scale for Depression (HAM-D), and Hamilton Rating Scale for Anxiety (HAM-A) [29-31]. Twenty patients were drug naïve, and 3 were on medication at the time of fMRI scanning. One of the 3 patients was taking 100 mg/day fluoxetine, another was taking 20 mg/day escitalopram, and the third was taking 100 mg/day sertraline. The exclusion criteria for both HCs and patients included any history of head injury, substance abuse (except smoking), seizure disorder, serious medical illness, and mental retardation [intelligent quotient (IQ)<70].
This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of SNUH (H-1902-142-101). Each subject received a complete description of the study and provided written informed consent before participation. For the minors who participated in this study, informed consent was obtained from both the participants themselves and their parents.
Functional and structural images were obtained with a 3.0-tesla Trio MRI scanner (Siemens Magnetom Trio, Erlangen, Germany) with a 12-channel head coil. T1-weighted structural images were acquired with the following parameters: echo time (TE)=1.89 ms; repetition time (TR)=1670 ms; field of view (FOV)=250 mm; flip angle=9°; matrix=256×256; voxel size=1.0×0.98×0.98 mm3; and 208 slices. Resting-state fMRI images were acquired with the following parameters: TE=30 ms; TR=3,500 ms; FOV=240 mm; flip angle=90°; matrix=128×128; voxel size=1.9×1.9×3.5 mm3; and 35 slices. During the functional imaging session, subjects were instructed to relax and keep their eyes closed but not to fall asleep. A questionnaire was administered to the participants after the scan to ensure that they had not fallen asleep. We used head cushions and asked subjects to move as little as possible during image acquisition to minimize motion artifacts.
We preprocessed the brain images using the CONN toolbox version 18b (CONN18b; https://www.nitrc.org/projects/conn) implemented in the software package Statistical Parametric Mapping version 12 (SPM12; http://www.fil.ion. ucl.ac.uk/spm/software/spm12/). The first 4 functional images were discarded for initial signal stabilization. The remaining images were realigned to correct head motion, and exclusion criteria for excessive head motion (i.e., translation>2 mm and rotation>2° in any direction) were applied. After motion correction, the functional images were processed by slice-timing correction and subsequent coregistration to structural images in each subject. The structural images were segmented into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). Then, both functional and structural images were spatially normalized to the Montreal Neurological Institute (MNI) space and resampled to 2×2×2 mm3 voxels. The normalized functional data were spatially smoothened with a 6 mm full width at half-maximum (FWHM) Gaussian kernel [32]. The smoothed images underwent nuisance regression using the CompCor method and were also processed by linear detrending and temporal bandpass filtering (0.008-0.09 Hz) [33].
We created 3-mm spherical striatal seeds in the dorsal caudate (DC), ventral caudate (VC), nucleus accumbens (NAc), dorsal rostral putamen (DRP), dorsal caudal putamen (DCP), and ventral rostral putamen (VRP) bilaterally, referring to the coordinate information from previous studies (Table 1) [8,26]. Using the seed ROIs, we measured connectivity strength using Pearson’s bivariate correlation analysis between the BOLD time series of the ROIs and other voxels in the rest of the brain. An independent t-test was conducted to compare the connectivity strength between groups. We applied an uncorrected height threshold of p<0.005 and a false discovery rate (FDR)-corrected cluster-level threshold of p<0.05 to find clusters that differed between groups at or above a minimum significance level [34]. Furthermore, we used a Bonferroni correction to address a multiple comparison issue arising from the use of 12 striatal seeds [35]. Finally, a stringent FDR-corrected threshold of p<0.05/12 was set for statistically significant clusters. CONN18b was used for all the FC analyses.
Fisher’s exact or the independent t-test was used to compare demographic characteristics between OCD patients and HCs. We conducted Pearson’s correlation analysis between striatal FC values, which were found to be altered in OCD patients compared to HCs, and measures of symptom severity, such as Y-BOCS, HAM-D, and HAM-A scores, within OCD patients. Statistical significance was defined for each test as a p<0.05. All statistical analyses except the group comparisons of FC were conducted using IBM SPSS Statistics 23 (IBM Corp., Armonk, NY, USA).
There was no significant demographic difference between OCD patients and HCs. The clinical characteristics of the patients, including duration of illness and Y-BOCS, HAM-D, and HAM-A scores, were measured to facilitate interpretation of our results. We summarize the subjects’ demographic and clinical characteristics in Table 2.
FC between the left DCP and the right intracalcarine cortex (ICC) was stronger in OCD patients than in HCs, while FC between the left DCP and the left putamen was weaker in patients than in HCs. FCs between the right VRP and the right inferior frontal gyrus (IFG), left supramarginal gyrus (SMG), right postcentral gyrus (PCG) and right SMG/angular gyrus (AG) were stronger in patients than in controls. The details of the significant regions are summarized in Table 3 and Figure 1. The striatal FC results with significant group differences before Bonferroni correction are presented in Supplementary Table 1 (in the online-only Data Supplement).
We found a negative correlation between the FC of the right VRP with the right SMG/AG and Y-BOCS compulsion scores (r=-0.420, p=0.046) (Figure 2A) in patients with OCD. In addition, connectivity between the right VRP and right PCG in OCD patients had a significant negative correlation with HAM-A scores (r=-0.542, p=0.008) (Figure 2B).
In the current study, we examined resting-state corticostriatal FC alterations and their relationship with symptom severity using a fine 12-seed striatal parcellation in OCD patients. We found that the FC values of the left DCP and right VRP with extensive cortical regions including the ICC, IFG, SMG/AG, and PCG were significantly stronger in patients than in HCs. Moreover, the FC values between the right VRP and right SMG/AG and between the right VRP and right PCG were negatively correlated with scores on the compulsive subscale of the Y-BOCS and on the HAM-A, respectively.
Strengthened FC between the right VRP and right SMG/AG and right PCG in OCD patients was a notable finding of the present study. The SMG/AG is responsible for a number of cognitive functions that are impaired in OCD patients, including visuospatial cognition, attention, awareness, and conflict resolution [36-38]. The PCG is a brain region belonging to the sensorimotor network, and it has been established that most patients suffering from OCD also have significant impairment of sensorimotor functions such as sensory gating [39-43]. Furthermore, numerous prior studies have demonstrated that patients with OCD have greater GM volume, gyrification, and metabolic rates than HCs in both the SMG/AG and the PCG [44-51]. These previous findings corroborate our present finding that alterations in the FC of the SMG/AG and PCG are associated with the pathophysiology of OCD. However, both FC values were negatively correlated with two measures of clinical symptom severity: Y-BOCS compulsion scores and HAM-A scores. These negative correlations may imply that the strengthened FC observed in OCD patients may be involved in a compensatory mechanism for obsessive-compulsive symptoms rather than contribute to the pathogenesis. The abovementioned increases in the volume and metabolic rate of the SMG/AG and PCG in patients with OCD also support this inference. However, since there is little scientific understanding of compensatory mechanisms in OCD, further research is required to prove such a claim.
Compared with HCs, patients with OCD exhibited strengthened FC between the right VRP and the right IFG in the present study. The IFG, especially the right IFG, is already known to be an important region for response inhibition [37,52,53]. A response inhibition deficit has been widely reported in patients with OCD using tasks such as go/no-go and stop-signal reaction time [54-58]. Therefore, it could be inferred that the IFG might be engaged in the pathophysiology of OCD, causing its FC with the striatum to be altered in OCD patients. However, the results of other studies searching for structural and functional alterations of the IFG in OCD were not consistent. Specifically, depending on the study, the GM volume was greater [49] or smaller [59,60] in OCD patients than in HCs. Resting-state FC between the IFG and ventral putamen was weaker in patients with OCD than in HCs [20]. Thus, the IFG has not yet been sufficiently investigated in OCD, despite the importance of its function, response inhibition. Thus, further studies should be carried out to specify the role and clinical significance of this region in OCD.
We also revealed that patients with OCD show stronger FC between the DCP and ICC than HCs do. In recent studies, the role of the parieto-occipital regions in OCD pathophysiology has received much attention. Such studies have claimed that the occipital regions are associated with deficits in visuospatial processing, which are consistently shown in OCD patients [61-63]. Thus, the ICC, part of the primary visual cortex, could be altered in patients with OCD. Indeed, several papers have reported structural and functional changes in the calcarine cortex in OCD patients [24,64-67]. Among those studies, Chen et al. [24] demonstrated strengthened FC between the caudate nucleus and calcarine sulcus in patients with OCD, which is in line with our result. Although a lack of knowledge restricts more specific interpretation of this finding, this study could provide additional information for future studies, given the increasing attention to the role of the occipital lobe in OCD.
One of the most unexpected findings of the current study was the lack of significant alterations in areas where such changes were previously reported, such as the OFC, ACC, and PFC. This discrepancy may be attributed to our patients’ clinical characteristics, which are significantly different from those of subjects in previous studies. In most preceding studies, the mean duration of illness was more than 10 years (Supplementary Table 2 in the online-only Data Supplement). In contrast, the mean duration of illness in our patients was 6.68 years. Recently, several studies have shown that the progression of OCD may accompany structural changes in the brain due to the neuroplasticity of the human brain [16,59,68-73]. Therefore, the comparatively short duration of illness in our patients could be a possible explanation for the inconsistency. In addition, Chen et al. [24], whose patients have a shorter mean duration of illness than our patients (5.54 years), reported results to ours. Those researchers were also unable to observe any significant FC alteration with commonly reported areas, but they found stronger FCs with cortical regions outside the traditional CSTC circuit, such as the temporal gyrus, calcarine sulcus, and PCG. These results support the inference about the relationship between our inconsistent results and the duration of illness. Furthermore, unlike most previous studies, we applied the Bonferroni correction for rigorous statistical analysis. This methodological difference might also cause the inconsistency in results. Indeed, when we did not correct for multiple comparisons, some results similar to previous findings were obtained, such as stronger FC between the right DC and subcallosal cortex/OFC in OCD patients than in HCs.
When interpreting the results of the present study, one must further consider several issues. First, 3 out of 23 patients were taking selective serotonin reuptake inhibitors (SSRIs). It is well known that psychotropic drugs, including SSRIs, can affect the FC pattern of the brain [74,75]. However, there have been several reports that SSRIs tend to normalize the pathological changes in patients’ brains [76-80]. Thus, medication is at least unlikely to increase the risk of false positive errors. The small number of subjects could be another limitation of this study. A small sample size lowers the reproducibility and reliability of findings [81]. If a future study with a large number of subjects is conducted, more accurate and reliable results can be obtained regarding the striatal FC alterations in OCD.
In conclusion, although we could not replicate the reported results of Posner et al. [28] and other previous studies using a fine striatal parcellation with 12 seeds, we discovered significantly altered FC between the striatal subregions and extensive cortical regions in patients with OCD, even with a stringent Bonferroni correction for multiple comparisons. In addition, the altered FC of the striatal subregions showed a significant relationship with the severity of compulsive and anxiety symptoms in patients with OCD. Therefore, the findings of the present study suggest that altered striatal FC could be a neural correlate of symptom severity in OCD patients. We expect that these findings will provide an improved understanding of the role of striatal FC in the pathophysiology of OCD. Future studies with large sample sizes and medication-naïve patients are necessary to support the results of the current study.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2019.0206.
ACKNOWLEDGEMENTS
Funding was provided by the Basic Science Research Program and the Basic Research Laboratory Program through the National Research Foundation of Korea (NRF) (Grant no. 2019R1A2B5B03100844 and 2018R1A4A1025891).
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Minah Kim, Junha Park, Jun Soo Kwon. Data curation: Taekwan Kim, Tae Young Lee. Formal analysis: Junha Park, Taekwan Kim, Minah Kim. Funding acquisition: Jun Soo Kwon. Investigation: Junha Park, Taekwan Kim, Tae Young Lee, Minah Kim, Jun Soo Kwon. Methodology: Junha Park, Taekwan Kim. Project administration: Minah Kim. Resources: Jun Soo Kwon. Software: Junha Park. Supervision: Minah Kim. Validation: Minah Kim, Tae Young Lee, Jun Soo Kwon. Visualization: Junha Park. Writing—original draft: Junha Park. Writing—review & editing: Minah Kim.
Figure 1.
t-statistic maps of the significant FC of the left DCP (A) and right VRP (B) in patients with OCD compared to healthy controls. The FC between the left DCP and the right intracalcarine cortex was stronger in patients with OCD than in healthy controls (red through yellow; A-1), whereas the opposite was true of the FC between the left DCP and the left putamen (shades of blue; A-2). The FC values between the right VRP and the right inferior frontal gyrus (B-1), left supramarginal gyrus (B-2), right postcentral gyrus (B-3) and right supramarginal/angular gyrus (B-4) were stronger in patients with OCD (red through yellow). All clusters survived false discovery rate and Bonferroni correction, with p-values<0.05/12. FC: functional connectivity, DCP: dorsal caudal putamen, VRP: ventral rostral putamen, OCD: obsessivecompulsive disorder.
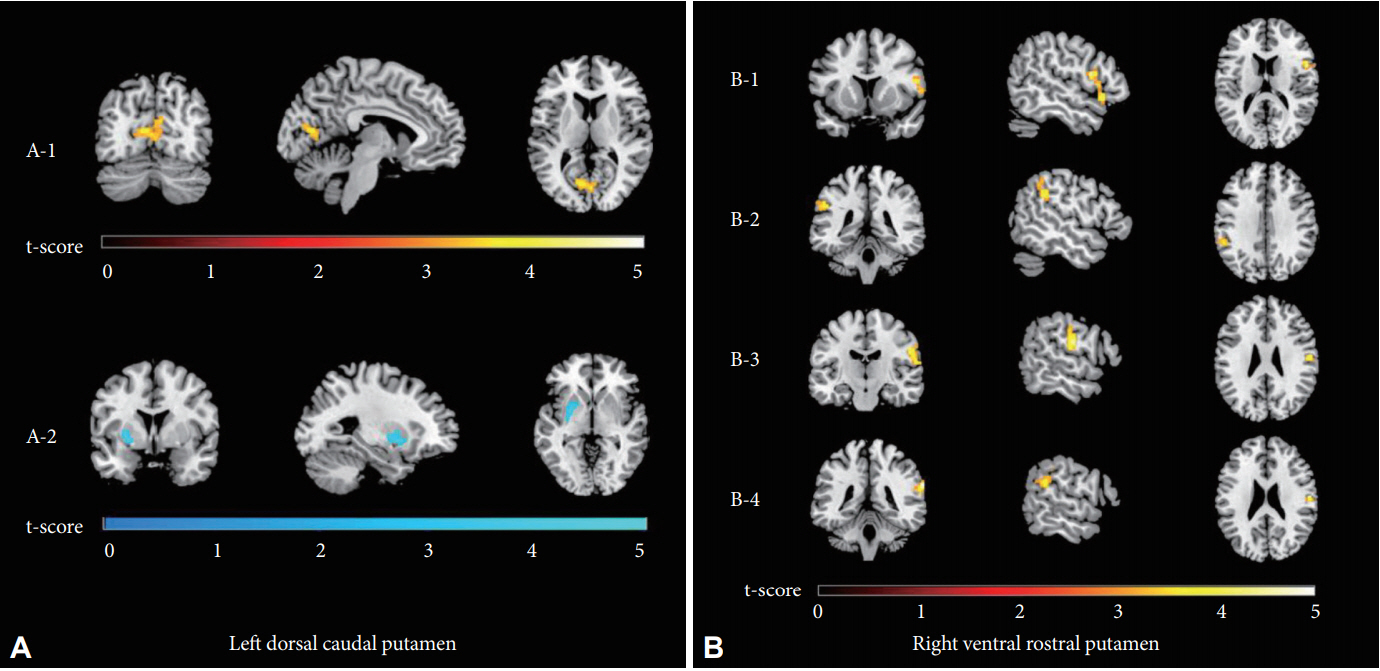
Figure 2.
Correlation analysis between the FC of the significant brain regions and clinical variables in patients with OCD. (A) The FC from the right VRP to the right supramarginal/angular gyrus (SMG/AG) was negatively correlated with scores on the compulsive subscale of the Y-BOCS in patients with OCD. (B) The FC from the right VRP to the right PCG was negatively correlated with scores on the HAM-A in patients with OCD. FC: functional connectivity, OCD: obsessive-compulsive disorder, VRP: ventral rostral putamen, Y-BOCS: Yale-Brown Obsessive Compulsive Scale, PCG: postcentral gyrus, HAM-A: Hamilton Rating Scale for Anxiety.
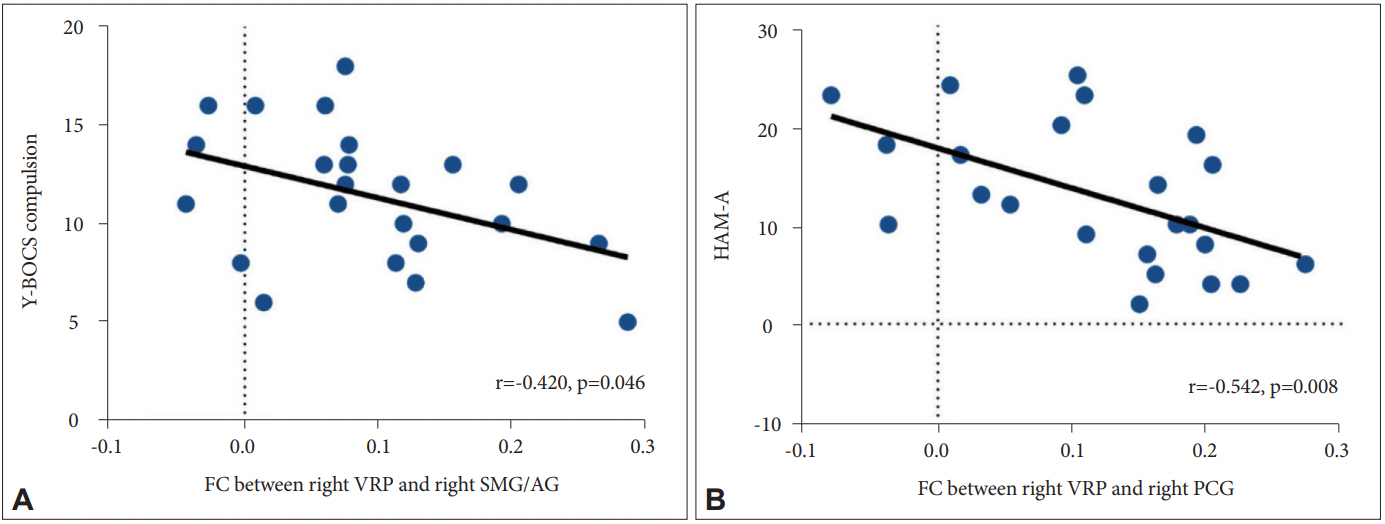
Table 1.
MNI coordinates of 12 striatal subregion seeds
Table 2.
Demographic and clinical characteristics of subjects
| OCD (N=23) | HCs (N=23) | Statistics*(t or χ2) | p-value | |
|---|---|---|---|---|
| Age (years) | 24.74±5.42 | 22.57±3.48 | -1.62 | 0.113 |
| Gender (male/female) | 19/4 | 19/4 | 0.00 | 1.000 |
| Handedness (left/right) | 1/22 | 1/22 | 0.00 | 1.000 |
| IQ | 110.39±15.61 | 109.87±16.53 | -0.11 | 0.913 |
| Education years (years) | 13.70±1.96 | 13.22±3.46 | 0.58 | 0.568 |
| Onset age (years) | 18.05±6.33 | - | - | - |
| Duration of illness (years) | 6.68±4.89 | - | - | - |
| Y-BOCS score | ||||
| Total | 23.43±6.79 | - | - | - |
| Obsession | 12.00±4.00 | - | - | - |
| Compulsion | 11.43±3.42 | - | - | - |
| HAM-D | 12.61±6.89 | - | - | - |
| HAM-A | 13.00±7.08 | - | - | - |
Table 3.
Group comparisons of striatal functional connectivity between OCD patients and HCs
REFERENCES
1. Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010;15:53-63.



2. Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 2012;16:43-51.


3. Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci 2014;15:410-424.



4. Koran LM, Thienemann M, Davenport R. Quality of life for patients with obsessive-compulsive disorder. Am J Psychiatry 1996;153:783-788.


5. Cavallaro R, Cavedini P, Mistretta P, Bassi T, Angelone SM, Ubbiali A, et al. Basal-corticofrontal circuits in schizophrenia and obsessive-compulsive disorder. Biol Psychiatry 2003;54:437-443.


6. Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science 2013;340:1234-1239.



7. Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 2007;448:894-900.




8. Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000;23:563-586.


9. Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state fMRI study. Cereb Cortex 2008;18:2735-2747.



10. Jung WH, Jang JH, Park JW, Kim E, Goo EH, Im OS, et al. Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state fMRI. PLoS One 2014;9:e106768



11. Lambrecq V, Rotgé JY, Guehl D, Bardinet E, Machado S, Cuny E, et al. Lesions in the associative striatum improve obsessive-compulsive disorder. Biol Psychiatry 2009;65:e11-e13.


12. Postle BR, D’Esposito M. Spatial working memory activity of the caudate nucleus is sensitive to frame of reference. Cogn Affect Behav Neurosci 2003;3:133-144.



13. Garavan H, Hester R, Murphy K, Fassbender C, Kelly C. Individual differences in the functional neuroanatomy of inhibitory control. Brain Res 2006;1105:130-142.


14. Crottaz-Herbette S, Anagnoson RT, Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. NeuroImage 2004;21:340-351.


15. Scarone S, Colombo C, Livian S, Abbruzzese M, Ronchi P, Locatelli M, et al. Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Res 1992;45:115-121.


16. Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry 2004;61:720-730.


17. Kang DH, Kim JJ, Choi JS, Kim YI, Kim CW, Youn T, et al. Volumetric investigation of the frontal-subcortical circuitry in patients with obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2004;16:342-349.


18. Riffkin J, Yücel M, Maruff P, Wood SJ, Soulsby B, Olver J, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Res 2005;138:99-113.


19. Fan Q, Yan X, Wang J, Chen Y, Wang X, Li C, et al. Abnormalities of white matter microstructure in unmedicated obsessive-compulsive disorder and changes after medication. PLoS One 2012;7:e35889



20. Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, et al. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry 2009;66:1189-1200.


21. Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishimura T, et al. Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry 2011;26:463-469.


22. Harrison BJ, Pujol J, Cardoner N, Deus J, Alonso P, López-Solà M, et al. Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry 2013;73:321-328.


23. Vaghi MM, Vértes PE, Kitzbichler MG, Apergis-Schoute AM, Van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry 2017;81:708-717.



24. Chen Y, Juhás M, Greenshaw AJ, Hu Q, Meng X, Cui H, et al. Abnormal resting-state functional connectivity of the left caudate nucleus in obsessive-compulsive disorder. Neurosci Lett 2016;623:57-62.


25. Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 2006;16:1508-1521.



26. Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 2011;69:847-856.


27. Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 2015;72:5-13.



28. Posner J, Marsh R, Maia TV, Peterson BS, Gruber A, Simpson HB. Reduced functional connectivity within the limbic cortico-striato-thalamo-cortical loop in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp 2014;35:2852-2860.


29. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46:1006-1011.


32. Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RS. Spatial registration and normalization of images. Hum Brain Mapp 1995;3:165-189.

33. Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage 1998;7:119-132.


34. Joshi G, Anteraper SA, Patil KR, Semwal M, Goldin RL, Furtak SL, et al. Integration and segregation of default mode network resting-state functional connectivity in transition-age males with high-functioning autism spectrum disorder: a proof-of-concept study. Brain Connect 2017;7:558-573.



35. Bland JM, Altman DG. Statistics notes: multiple significance tests: the Bonferroni method. BMJ 1995;310:170



37. Zhang R, Geng X, Lee TMC. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct Funct 2017;222:3973-3990.




38. Studer B, Cen D, Walsh V. The angular gyrus and visuospatial attention in decision-making under risk. NeuroImage 2014;103:75-80.


39. Doucet GE, Bassett DS, Yao N, Glahn DC, Frangou S. The role of intrinsic brain functional connectivity in vulnerability and resilience to bipolar disorder. Am J Psychiatry 2017;174:1214-1222.



40. Rossi S, Bartalini S, Ulivelli M, Mantovani A, Di Muro A, Goracci A, et al. Hypofunctioning of sensory gating mechanisms in patients with obsessive-compulsive disorder. Biol Psychiatry 2005;57:16-20.


41. Ahmari SE, Risbrough VB, Geyer MA, Simpson HB. Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacology 2012;37:1216-1223.




42. De Leeuw AS, Oranje B, Van Megen HJ, Kemner C, Westenberg HG. Sensory gating and sensorimotor gating in medication-free obsessivecompulsive disorder patients. Int Clin Psychopharmacol 2010;25:232-240.


43. Russo M, Naro A, Mastroeni C, Morgante F, Terranova C, Muscatello MR, et al. Obsessive-compulsive disorder: a “sensory-motor” problem? Int J Psychophysiol 2014;92:74-78.


44. Subirà M, Sato J, Alonso P, do Rosário M, Segalàs C, Batistuzzo M, et al. Brain structural correlates of sensory phenomena in patients with obsessive-compulsive disorder. J Psychiatry Neurosci 2015;40:232-240.




45. Göttlich M, Krämer UM, Kordon A, Hohagen F, Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp 2014;35:5617-5632.



46. Tang W, Zhu Q, Gong X, Zhu C, Wang Y, Chen S. Cortico-striatothalamo-cortical circuit abnormalities in obsessive-compulsive disorder: a voxel-based morphometric and fMRI study of the whole brain. Behav Brain Res 2016;313:17-22.


47. Le Jeune F, Vérin M, N’Diaye K, Drapier D, Leray E, Du Montcel ST, et al. Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry 2010;68:1016-1022.


48. Fan Q, Palaniyappan L, Tan L, Wang J, Wang X, Li C, et al. Surface anatomical profile of the cerebral cortex in obsessive-compulsive disorder: a study of cortical thickness, folding and surface area. Psychol Med 2013;43:1081-1091.


49. Tan L, Fan Q, You C, Wang J, Dong Z, Wang X, et al. Structural changes in the gray matter of unmedicated patients with obsessive-compulsive disorder: a voxel-based morphometric study. Neurosci Bull 2013;29:642-648.




50. Chen Y, Meng X, Hu Q, Cui H, Ding Y, Kang L, et al. Altered restingstate functional organization within the central executive network in obsessive-compulsive disorder. Psychiatry Clin Neurosci 2016;70:448-456.


51. Hirose M, Hirano Y, Nemoto K, Sutoh C, Asano K, Miyata H, et al. Relationship between symptom dimensions and brain morphology in obsessive-compulsive disorder. Brain Imaging Behav 2017;11:1326-1333.



52. Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cognit Sci 2004;8:170-177.

53. Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A 2010;107:6106-6111.



54. Penadés R, Catalán R, Andrés S, Salamero M, Gastó C. Executive function and nonverbal memory in obsessive-compulsive disorder. Psychiatry Res 2005;133:81-90.


55. Penadés R, Catalán R, Rubia K, Andrós S, Salamero M, Gastó C. Impaired response inhibition in obsessive compulsive disorder. Eur Psychiatry 2007;22:404-410.


56. Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 2008;32:525-549.


57. Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM. Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Res 2002;110:165-174.


58. Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry 2007;164:335-338.



59. De Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchón JM, et al. Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry 2014;171:340-349.


60. Hou J, Song L, Zhang W, Wu W, Wang J, Zhou D, et al. Morphologic and functional connectivity alterations of corticostriatal and default mode network in treatment-naïve patients with obsessive-compulsive disorder. PLoS One 2013;8:e83931



61. GonÇalves ÓF, Marques TR, Lori NF, Sampaio A, Branco MC. Obsessive-compulsive disorder as a visual processing impairment. Med Hypotheses 2010;74:107-109.


62. Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, et al. Disorganization of anatomical connectivity in obsessive compulsive disorder: a multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiol Dis 2010;37:468-476.


63. Piras F, Piras F, Chiapponi C, Girardi P, Caltagirone C, Spalletta G. Widespread structural brain changes in OCD: a systematic review of voxel-based morphometry studies. Cortex 2015;62:89-108.


64. Moon CM, Kim BC, Jeong GW. Associations of neurofunctional, morphometric and metabolic abnormalities with clinical symptom severity and recognition deficit in obsessive-compulsive disorder. J Affect Disord 2018;227:603-612.


65. Yang XY, Sun J, Luo J, Zhong ZX, Li P, Yao SM, et al. Regional homogeneity of spontaneous brain activity in adult patients with obsessivecompulsive disorder before and after cognitive behavioural therapy. J Affect Disord 2015;188:243-251.


66. Ping L, Su-Fang L, Hai-Ying H, Zhang-Ye D, Jia L, Zhi-Hua G, et al. Abnormal spontaneous neural activity in obsessive-compulsive disorder: a resting-state functional magnetic resonance imaging study. PLoS One 2013;8:e67262



67. Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, et al. White matter abnormalities in obsessive-compulsive disorder. Arch Gen Psychiatry 2005;62:782-790.


68. Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am J Psychiatry 2017;174:60-69.


69. Boedhoe PS, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA obsessive-compulsive disorder working group. Am J Psychiatry 2018;175:453-462.


70. Fontenelle LF, Yücel M. A clinical staging model for obsessive-compulsive disorder: is it ready for prime time? EClinicalMedicine 2019;7:65-72.



71. Fouche JP, du Plessis S, Hattingh C, Roos A, Lochner C, Soriano-Mas C, et al. Cortical thickness in obsessive-compulsive disorder: multisite mega-analysis of 780 brain scans from six centres. Br J Psychiatry 2017;210:67-74.


72. Atmaca M, Yildirim H, Ozdemir H, Ozler S, Kara B, Ozler Z, et al. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:1283-1286.


73. Yoo SY, Roh MS, Choi JS, Kang DH, Ha TH, Lee JM, et al. Voxel-based morphometry study of gray matter abnormalities in obsessive-compulsive disorder. J Korean Med Sci 2008;23:24-30.



74. Schwarz AJ, Gozzi A, Reese T, Bifone A. In vivo mapping of functional connectivity in neurotransmitter systems using pharmacological MRI. Neuroimage 2007;34:1627-1636.


75. Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, et al. Antidepressant effect on connectivity of the mood-regulating circuit: an fMRI study. Neuropsychopharmacology 2005;30:1334-1344.



76. Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry 2013;70:619-629.


77. Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology 2010;35:317-336.



78. Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry 2005;57:901-910.


79. Kang DH, Kwon JS, Kim JJ, Youn T, Park HJ, Kim MS, et al. Brain glucose metabolic changes associated with neuropsychological improvements after 4 months of treatment in patients with obsessive-compulsive disorder. Acta Psychiatr Scand 2003;107:291-297.









