Modified Criteria for Diagnosing “Cognitive Frailty”
Article information
Abstract
The concept of cognitive frailty has recently been proposed by an International Consensus Group as the presence of physical frailty and cognitive impairment [defined using the Clinical Dementia Ratings (CDR)=0.5], without concurrent dementia. However, CDR is difficult to implement and not often available in epidemiologic studies or busy clinical settings, and an alternative to CDR is required. We suggest an alternative definition of cognitive frailty as: 1) physical frailty, 2) more than 1.5 standard deviation below the mean for age-, gender-, and education-adjusted norms on any cognitive function test (e.g., the Montreal Cognitive assessment test, the Alzheimer’s disease assessment scale-cognitive subscale, verbal learning test, Digit Span, Boston Naming Test, Trail Making Test, and Frontal Assessment Battery), and 3) no dependency in instrumental activities of daily living. The redefined criteria for cognitive frailty would be more feasible to implement and thus more applicable in epidemiologic studies and busy clinical settings.
LINK BETWEEN PHYSICAL FRAILTY AND COGNITIVE IMPAIRMENT
With increasing life expectancy, frailty and cognitive impairment are being recognized as major threats to healthy aging and quality of life. Frailty in late life predisposes the individual to increased vulnerability to stressors, elevating the risk of disability, institutionalization, and mortality [1]. Physical frailty is quite prevalent in older adults, with a recent systematic review reporting 9.9% of community-dwelling adults aged 65 years and older to have the condition [2]. With age-related cognitive decline, cognitive impairment that does not reach the threshold of dementia is commonly observed in older people, with an increased risk for progression to dementia, contributing to increased disability and healthcare costs [3].
Physical frailty and cognitive impairment often co-occur, with the two conditions being closely interrelated. About 20% of physically frail individuals living in the community are said to be cognitively impaired [4]. Several cohort studies have reported that physical frailty predicts the onset of cognitive decline and incident dementia [5-7]. Cognitive impairment has also been observed to predict physical frailty [8]. Moreover, physical frailty and cognitive impairment appear to reinforce each other, resulting in detrimental outcomes. Frailty when combined with mild cognitive impairment or dementia elevates the risk of poor outcomes [9].
Based on the results of these studies, a concept of “cognitive frailty” has been recently proposed by the International Consensus Group on Cognitive Frailty, organized by the International Academy on Nutrition and Aging (IANA) and the International Association of Gerontology and Geriatrics (IAGG) in France [8]. According to the International Consensus Group, cognitive frailty is defined as the 1) presence of physical frailty and cognitive impairment [Clinical Dementia Rating (CDR)=0.5] and 2) exclusion of concurrent dementia (Figure 1) [10].
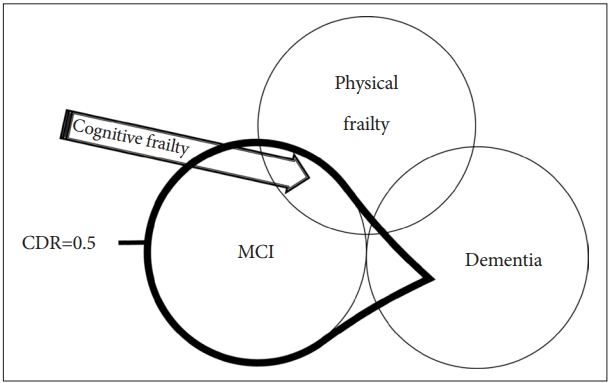
Definition of cognitive frailty by IANA/IAGG International Consensus Group. Cognitive frailty is defined as the presence of physical frailty and cognitive impairment (CDR=0.5) and the exclusion of concurrent dementia. CDR=0.5 includes mild cognitive impairment (MCI) and some dementia, but the exclusion of concurrent dementia limits the boundary to MCI. Cognitive frailty corresponds to the area indicated by the arrow.
The CDR is widely accepted in the clinical setting as a reliable and valid tool for assessing the severity of dementia [11]. However, the CDR is often not available or difficult to implement in epidemiologic studies or busy clinical settings. It is important to note that the CDR is a clinical protocol composed of semi-structured interviews where a clinician or neuropsychologist obtains information from the patient and proxy respondent, and then rates the cognitive performance of the patient in six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Furthermore, epidemiologic field surveys sometimes entail recognizing and excluding possible dementia patients in the absence of dementia specialists or neuropsychologists. Therefore, the CDR may not be appropriate for use in epidemiologic studies because of its complexity in measurement and difficulty to implement in the field settings. Thus, we suggest alternative criteria to CDR 0.5 and exclusion of dementia for diagnosing cognitive frailty.
AN ALTERNATIVE TO THE CDR SCORE OF 0.5
Based on the definition by the International Consensus Group of IANA and IAGG, a CDR score 0.5 and the exclusion of concurrent dementia correspond to the concept of mild cognitive impairment (MCI). Many investigators have used a CDR score of 0.5 to define MCI. However, a CDR score of 0.5 is not equivalent to the diagnosis of MCI [12]. In one study, 39.7% of people with a CDR score of 0.5 were found to be demented [13]. Therefore, the diagnosis of MCI and the exclusion of dementia cannot be determined with the CDR score alone.
How can we devise an alternative to the CDR score of 0.5 and the exclusion of concurrent dementia? Petersen’s original criteria of MCI used neuropsychological (NP) test performance and operationalized MCI as a score of more than 1.5 standard deviations below that of age-appropriate norms on a measure of episodic memory with performance within the normal range on nonmemory tests [14]. Using a similar approach, the International Working Group on MCI has adapted Petersen’s criteria to take into account differing patterns of cognitive test performance and allowed either memory or nonmemory impairment and single cognitive domain or multiple impaired domains [15].
As to the cognitive assessment for cognitive frailty, the International Consensus Group on Cognitive Frailty by IANA and IAGG have already suggested comprehensive cognitive assessment exploring memory performance as well as other cognitive functions (i.e., executive functions). The panel have also suggested several cognitive tests such as speed of processing test, the Montreal Cognitive assessment (MoCA) test, the Mini Mental state Examination (MMSE) and the Alzheimer’s disease assessment scale-cognitive subscale (ADAS-Cog) [10].
However, the MMSE had low sensitivity (66.01%) for MCI by traditionally accepted cut-off points set at 26/27 [16]. On the other hand, for MoCA test, the best cut-off point is 24/25 with sensitivity of 80.48% and specificity of 81.19% [17].
Among cognitive abilities, the frontal lobe function is important and needs to be assessed for diagnosis of cognitive frailty. This is because cognitively frail individuals might demonstrate significantly more impairments in executive functions than individuals with cognitive impairment but without physical frailty [18]. Executive functions primarily affected in the cognitive frailty were processing speed, selective attention, and mental flexibility, reflecting sub-cortico-frontal cognitive patterned impairments [18].
EXCLUSION OF DEMENTIA
To diagnose cognitive frailty, we should exclude dementia as well. In 2011, a working group charged by the National Institute on Aging and the Alzheimer’s Association revised the diagnostic criteria for MCI [19] (Table 1). The criteria for dementia stipulate that cognitive impairment must be present in two or more domains and must interfere with the abilities to function in daily activities [19,20]. A key criterion for MCI is the absence of dementia. Thus, individuals have to experience cognitive impairment while keeping their functioning abilities intact. Therefore, the preservation of functional activities is essential to differentiating MCI from dementia [15,21].
NEWLY SUGGESTED CRITERIA OF COGNITIVE FRAILTY
Based on the above reviews, we propose the new criteria of cognitive frailty as follows (Figure 2).
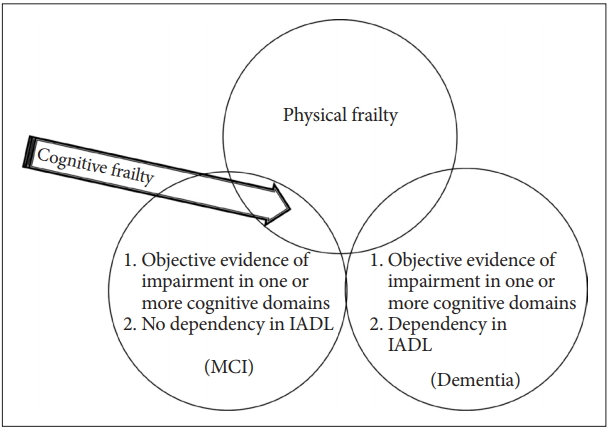
Newly suggested definition of cognitive frailty for epidemiologic studies. Any cognitive function test < -1.5 SD of the age-, gender-, and education-adjusted norms mean MCI or Dementia. Dependency in IADL encompasses dementia. The newly suggested definition of cognitive frailty (arrow) is physical frailty and any cognitive function test < -1.5 SD of the age-, gender-, and education-adjusted norm and no dependency in IADL. MCI: mild cognitive impairment, IADL: instrumental activities of daily living.
1) physical frailty; 2) 1.5 SD below the mean for age-, gender-, and education-adjusted norms on any cognitive functioning test (e.g., the MoCA, the ADAS-Cog, verbal learning test, Digit Span, Boston Naming Test, Trail Making Test, and Frontal Assessment Battery); and 3) no dependency in instrumental activities of daily living (IADL); Among IADL items, financial capacity (managing money), telephone use, responsibility for medication, and keeping appointments should be included because these items are particularly more complex tasks requiring high cognitive demands [18].
In conclusion, we propose a new definition of cognitive frailty for wider use in epidemiologic studies and busy clinical settings. The revised criteria need to be tested to determine its value in accurately assessing cognitive frailty and predicting its outcomes.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C3153).
