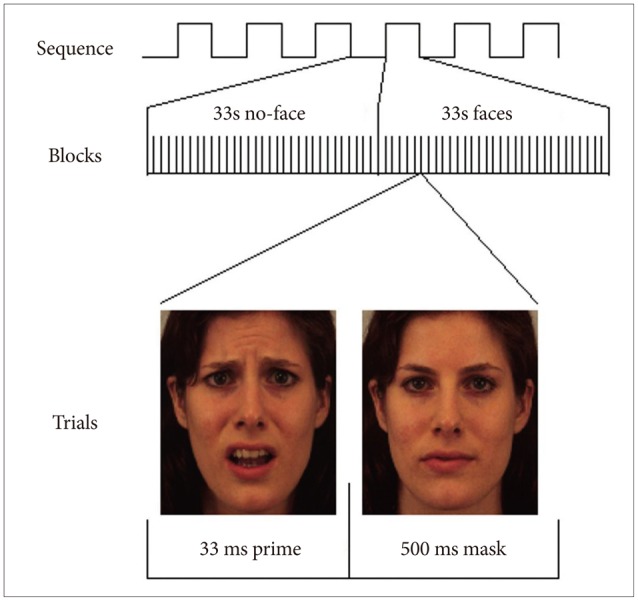
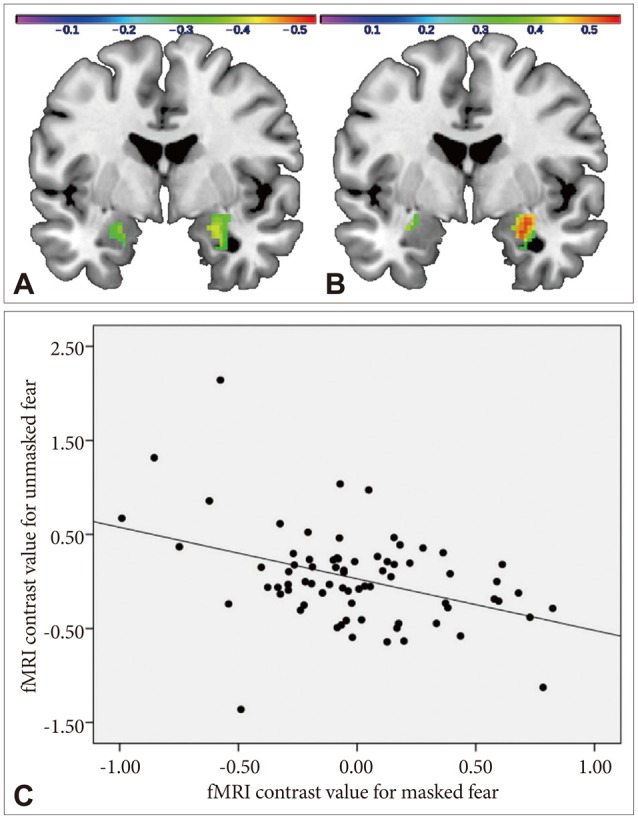
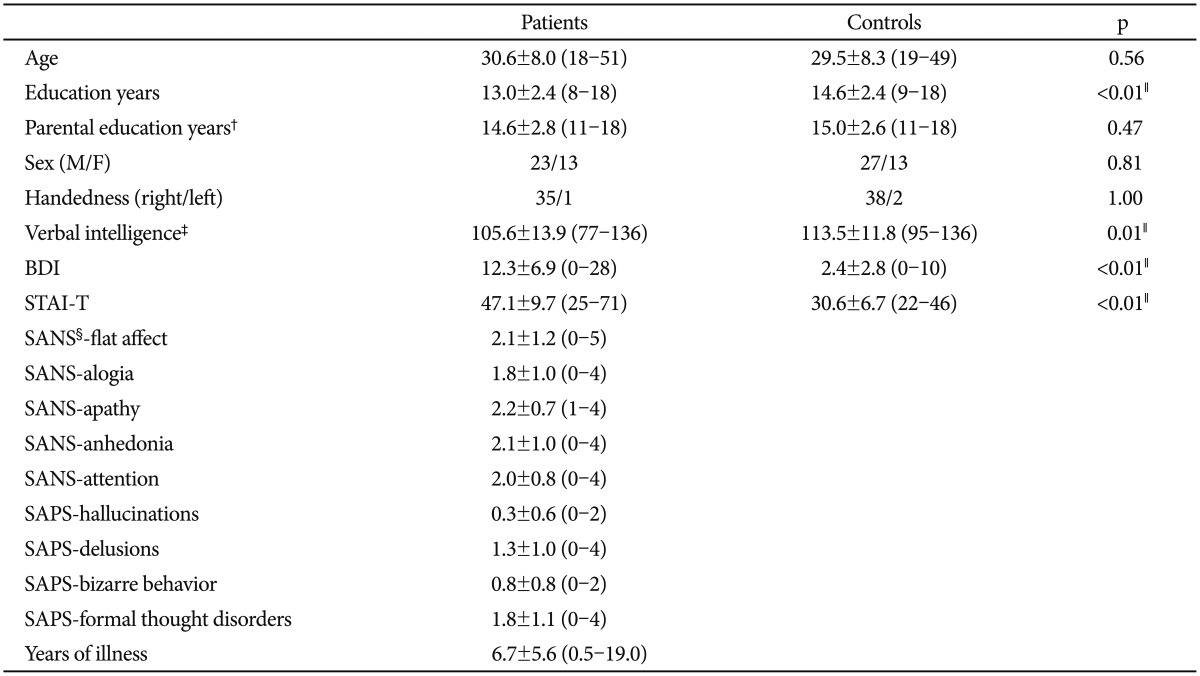
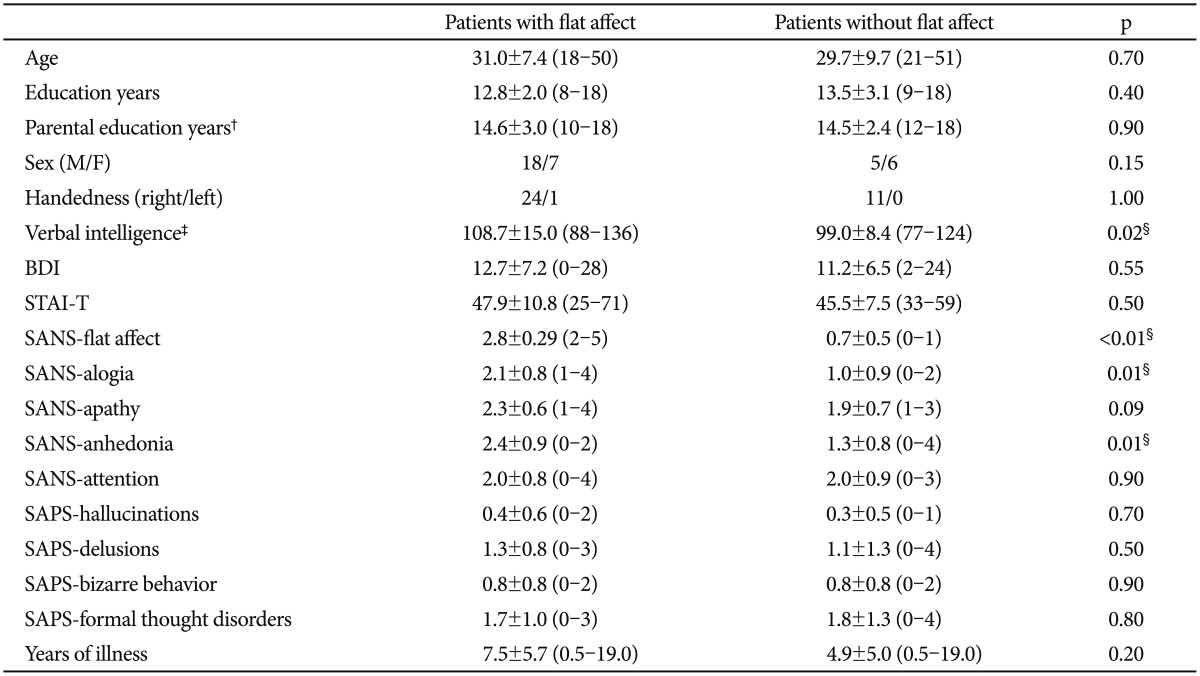
1. Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull 2008;34:819-834. PMID:
18579556.



2. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
3. Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol 2007;116:30-42. PMID:
17324014.


4. Herbener ES, Rosen C, Khine T, Sweeney JA. Failure of positive but not negative emotional valence to enhance memory in schizophrenia. J Abnorm Psychol 2007;116:43-55. PMID:
17324015.


5. Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol 1993;102:507-517. PMID:
8282918.


6. Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J Abnorm Psychol 1996;105:249-257. PMID:
8723006.


7. Flack WF, Laird JD, Cavallaro LA, Miller DR. Emotional Expression and Experience. A Psychosocial Perspective on Schizophrenia. In: Flack WF, Laird JD, editor. Emotions in Psychopathology. Oxford: Oxford University Press, 1998, p. 315-322.
8. Krause R, Steimer-Krause E, Hufnagel H. Expression and experience of affects in paranoid schizophrenia. Eur Rev Appl Psychol 1992;42:131-140.
9. Kohler CG, Turner TH, Bilker WB, Brensinger CM, Siegel SJ, Kanes SJ, et al. Facial emotion recognition in schizophrenia: intensity effects and error pattern. Am J Psychiatry 2003;160:1768-1774. PMID:
14514489.


10. Mandal MK, Pandey R, Prasad AB. Facial expressions of emotions and schizophrenia: review. Schizophr Bull 1998;24:399-412. PMID:
9718632.


11. Morrison RL, Bellack AS, Mueser KT. Deficits in facial-affect recognition and schizophrenia. Schizophr Bull 1988;14:67-83. PMID:
3291095.


12. Morris RW, Weickert CS, Loughland CM. Emotional face processing in schizophrenia. Curr Opin Psychiatry 2009;22:140-146. PMID:
19553867.


13. Alfimova MV, Abramova LI, Barhatova AI, Yumatova PE, Lyachenko GL, Golimbet VE. Facial affect recognition deficit as a marker of genetic vulnerability to schizophrenia. Span J Psychol 2009;12:46-55. PMID:
19476218.


14. Addington J, Penn D, Woods SW, Addington D, Perkins DO. Facial affect recognition in individuals at clinical high risk for psychosis. Br J Psychiatry 2008;192:67-68. PMID:
18174514.



15. Anticevic A, Van Snellenberg JX, Cohen RE, Repovs G, Dowd EC, Barch DM. Amygdala recruitment in Schizophrenia in response to aversive emotional material: a meta-analysis of neuroimaging Studies. Schizophr Bull 2012;38:608-621. PMID:
21123853.


16. Schneider F, Weiss U, Kessler C, Salloum JB, Posse S, Grodd W, et al. Differential amygdala activation in schizophrenia during sadness. Schizophr Res 1998;34:133-142. PMID:
9850979.


17. Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, et al. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry 2002;159:1992-1999. PMID:
12450947.


18. Johnstone PJ, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. Eur J Neurosci 2005;22:1221-1232. PMID:
16176365.


19. Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry 2003;53:1099-1122. PMID:
12814861.


20. Blasi G, Popolizio T, Taurisano P, Caforio G, Romano R, Di Giorgio A, et al. Changes in prefrontal and amygdala activity during olanzapine treatment in schizophrenia. Psychiatry Res 2009;173:31-38. PMID:
19428222.



21. Fernandez-Egea E, Parellada E, Lomena F, Falcon C, Pavia J, Mane A, et al. (18)FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2010;260:69-76. PMID:
19452191.


22. Kosaka H, Omori M, Murata T, Iidaka T, Yamada H, Okada T, et al. Differential amygdala response during facial recognition in patients with schizophrenia: an fMRI study. Schizophr Res 2002;57:87-95. PMID:
12165379.


23. Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, et al. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res 2006;82:153-162. PMID:
16377154.


24. Gur RE, Loughead J, Kohler CG, Elliott MA, Lesko K, Ruparel K, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry 2007;64:1356-1366. PMID:
18056543.


25. Lepage M, Sergerie K, Benoit A, Czechowska Y, Dickie E, Armony JL. Emotional face processing and flat affect in schizophrenia: Functional and structural neural correlates. Psychol Med 2011;41:1833-1844. PMID:
21284912.


26. Höschel K, Irle E. Emotional priming of facial affect identification in schizophrenia. Schizophr Bull 2001;27:317-327. PMID:
11354598.


27. Suslow T, Roestel C, Arolt V. Affective priming in schizophrenia with and without affective negative symptoms. Eur Arch Psychiatry Clin Neurosci 2003;253:292-300. PMID:
14714118.


28. Rauch AV, Reker M, Ohrmann P, Pedersen A, Bauer J, Dannlowski U, et al. Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Res Neuroimaging 2010;182:200-206. PMID:
20488680.

29. Murphy ST, Zajonc RB. Affect, cognition, and awareness: affective priming with optimal and suboptimal stimulus exposures. J Pers Soc Psychol 1993;64:723-739. PMID:
8505704.


30. Suslow T, Roestel C, Ohrmann P, Arolt V. The experience of basic emotions in patients with and without affective negative symptoms. Compr Psychiatry 2003;44:303-310. PMID:
12923708.


31. Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev 1977;84:1-66.

32. Moors A, de Houwer J. Automaticity: a theoretical and conceptual analysis. Psychol Bull 2006;132:297-326. PMID:
16536645.


33. Bargh JA. The Four Horsemen of Automaticity: Awareness, Intention, Efficiency, and Control in Social Cognition. In: Wyer RS, Srull TK, editor. Handbook of Social Cognition (Vol. 1). Hillsdale: Erlbaum, 1994, p. 1-40.
34. Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. SKID-I. Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe; 1997.
35. Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa; 1984.
36. Beck AT, Steer RA. Beck Depression Inventory: Manual. San Antonio, TX: Psychological Corporation Harcourt Brace Jovanovich; 1987.
37. Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory: STAI. Palo Alto: Consulting Psychologists Press; 1983.
38. Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Göttingen: Hogrefe; 1995.
39. Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces (KDEF). Stockholm: Karolinska Institute, Department of Clinical Neuroscience; 1998.
40. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273-289. PMID:
11771995.


41. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233-1239. PMID:
12880848.


42. Fakra E, Salgado-Pineda P, Delaveau P, Hariri A, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res 2008;100:191-205. PMID:
18234477.


43. Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res 1999;92:11-31. PMID:
10688157.


44. Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol 1962;17:827-838.

45. Meehl PE. Primary and secondary hypohedonia. J Abnorm Psychol 2001;110:188-193. PMID:
11261394.


46. Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev 2004;45:96-103. PMID:
15145620.


47. Varcin KJ, Bailey PE, Henry JD. Empathic deficits in schizophrenia: the potential role of rapid facial mimicry. J Int Neuropsychol Soc 2010;16:621-629. PMID:
20374674.