1. McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry 2020;77:201-210.


2. World Health Organization. ICD-10: international statistical classification of diseases and related health problems: 10th revision (2nd ed). Geneva: World Health Organization; 2004.
3. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed). Arlington: American Psychiatric Association; 2013.
8. Dennison CA, Legge SE, Pardiñas AF, Walters JTR. Genome-wide association studies in schizophrenia: recent advances, challenges and future perspective. Schizophr Res 2020;217:4-12.


9. O’Connell G, Lawrie SM, McIntosh AM, Hall J. Schizophrenia risk genes: implications for future drug development and discovery. Biochem Pharmacol 2011;81:1367-1373.


10. Barker V, Gumley A, Schwannauer M, Lawrie SM. An integrated biopsychosocial model of childhood maltreatment and psychosis. Br J Psychiatry 2015;206:177-180.


11. Engel GL. The clinical application of the biopsychosocial model. J Med Philos 1981;6:101-124.


14. Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 2006;163:521-528.


15. Land W. Allograft injury mediated by reactive oxygen species: from conserved proteins of Drosophila to acute and chronic rejection of human transplants. Part II: role of reactive oxygen species in the induction of the heat shock response as a regulator of innate. Transplant Rev 2003;17:31-44.

16. Zindel J, Kubes P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu Rev Pathol 2020;15:493-518.


17. Cochran AJ, Lu HF, Li PX, Saxton R, Wen DR. S-100 protein remains a practical marker for melanocytic and other tumours. Melanoma Res 1993;3:325-330.


19. Rickmann M, Wolff JR. S100 protein expression in subpopulations of neurons of rat brain. Neuroscience 1995;67:977-991.


20. Michetti F, Rende M, Lauriola L, Stolfi V, Calogero G, Cocchia D. Immunohistochemical localization of S-100-containing cells in non-nervous structures of the human eye. Cell Biol Int Rep 1986;10:765-773.


21. Li D, Li K, Chen G, Xia J, Yang T, Cai P, et al. S100B suppresses the differentiation of C3H/10T1/2 murine embryonic mesenchymal cells into osteoblasts. Mol Med Rep 2016;14:3878-3886.


23. Xiong Z, O’Hanlon D, Becker LE, Roder J, MacDonald JF, Marks A. Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp Cell Res 2000;257:281-289.


24. Gógl G, Alexa A, Kiss B, Katona G, Kovács M, Bodor A, et al. Structural basis of ribosomal S6 kinase 1 (RSK1) inhibition by S100B protein: modulation of the extracellular signal-regulated kinase (ERK) signaling cascade in a calcium-dependent way. J Biol Chem 2016;291:11-27.

25. Donato R. Calcium-independent, pH-regulated effects of S-100 proteins on assembly-disassembly of brain microtubule protein in vitro. J Biol Chem 1988;263:106-110.


27. Businaro R, Leone S, Fabrizi C, Sorci G, Donato R, Lauro GM, et al. S100B protects LAN-5 neuroblastoma cells against Abeta amyloid-induced neurotoxicity via RAGE engagement at low doses but increases Abeta amyloid neurotoxicity at high doses. J Neurosci Res 2006;83:897-906.

28. Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets 2018;22:263-277.


32. Yang M, Yang X, Wang S, Xu L, Ke S, Ding X, et al. HMGB1-induced endothelial cell pyroptosis is involved in systemic inflammatory response syndrome following radiofrequency ablation of hepatic hemangiomas. Am J Transl Res 2019;11:7555-7567.


34. Cuijpers P. Meta-analyses in mental health research. A practical guide. Amsterdam: VU University Amsterdam; 2016.
35. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963-974.


36. Hedges LV, Olkin I. Statistical methods for meta-analysis. San Diego: Academic Press; 1985.
37. Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-129.

39. Orwin RG. A fail-safe N for effect size in meta-analysis. J Educ Stat 1983;8:157-159.


40. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-1101.


42. Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89-98.

43. Şahin M, Aybek E. Jamovi: an easy to use statistical software for the social scientists. Int J Assess Tool Educ 2019;6:670-692.

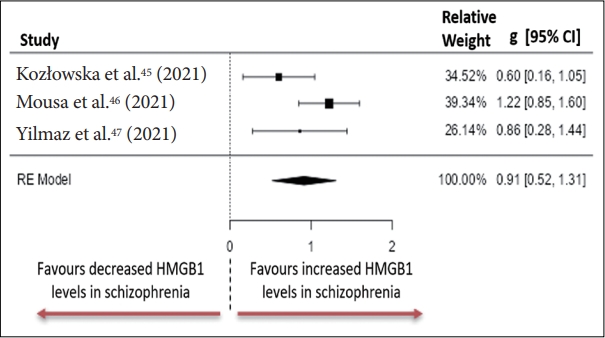
45. Kozłowska E, Brzezińska-Błaszczyk E, Agier J, Wysokiński A, Żelechowska P. Alarmins (IL-33, sST2, HMGB1, and S100B) as potential biomarkers for schizophrenia. J Psychiatr Res 2021;138:380-387.


48. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull 1988;24:97-99.
49. Andreasen NC. Scale for the assessment of positive symptoms (SAPS). Iowa: University of Iowa; 1984.
50. Iager AC, Kirch DG, Wyatt RJ. A negative symptom rating scale. Psychiatry Res 1985;16:27-36.


52. Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Neuron-specific enolase is unaltered whereas S100B is elevated in serum of patients with schizophrenia--original research and meta-analysis. Psychiatry Res 2009;167:66-72.


53. Steiner J, Schroeter ML, Schiltz K, Bernstein HG, Müller UJ, Richter-Landsberg C, et al. Haloperidol and clozapine decrease S100B release from glial cells. Neuroscience 2010;167:1025-1031.


54. Capuzzi E, Bartoli F, Crocamo C, Clerici M, Carrà G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: a meta-analysis. Neurosci Biobehav Rev 2017;77:122-128.


55. Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry 2009;65:481-488.


58. Tourjman V, Kouassi É, Koué MÈ, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res 2013;151:43-47.


59. Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. Changes in serum interleukin-2, -6, and -8 levels before and during treatment with risperidone and haloperidol: relationship to outcome in schizophrenia. J Clin Psychiatry 2004;65:940-947.


61. Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech 2003;60:614-632.


62. Tzoulaki I, Jarvelin MR, Hartikainen AL, Leinonen M, Pouta A, Paldanius M, et al. Size at birth, weight gain over the life course, and lowgrade inflammation in young adulthood: northern Finland 1966 Birth Cohort study. Eur Heart J 2008;29:1049-1056.


65. Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 2003;17:884-886.

66. Allsopp K, Read J, Corcoran R, Kinderman P. Heterogeneity in psychiatric diagnostic classification. Psychiatry Res 2019;279:15-22.


70. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007;64:1123-1131.


71. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349:1436-1442.


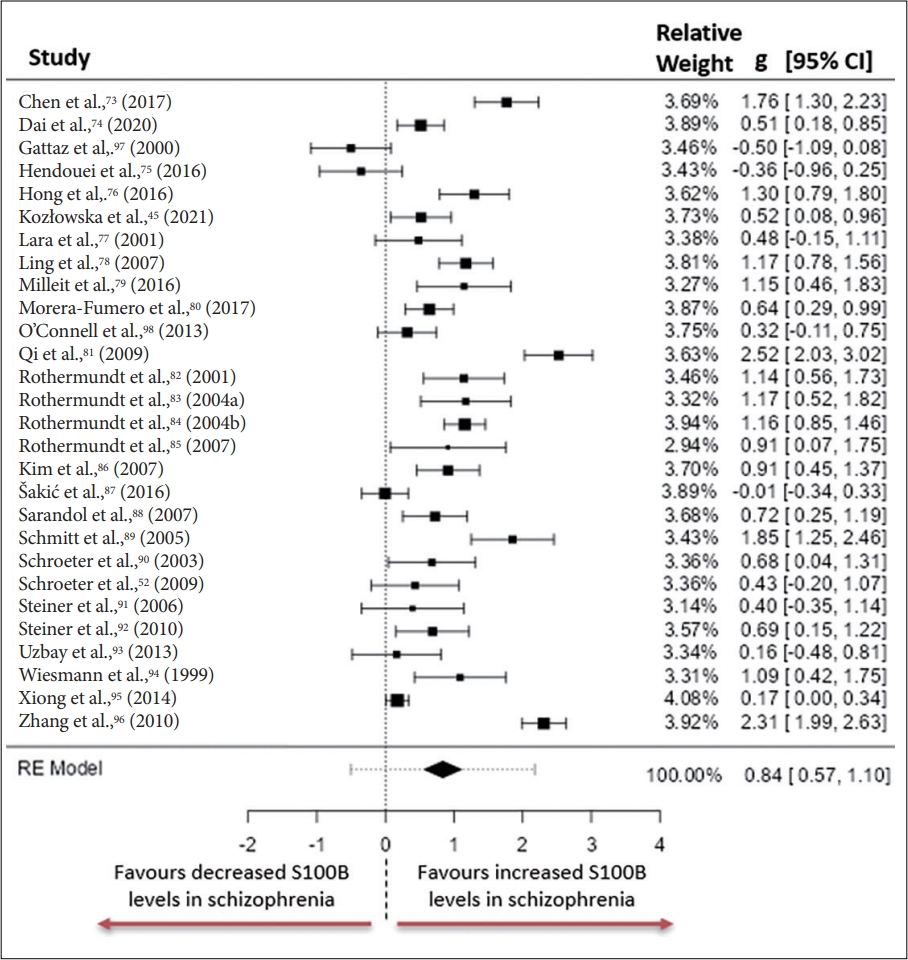
73. Chen S, Tian L, Chen N, Xiu M, Wang Z, Yang G, et al. Cognitive dysfunction correlates with elevated serum S100B concentration in drugfree acutely relapsed patients with schizophrenia. Psychiatry Res 2017;247:6-11.


75. Hendouei N, Hosseini SH, Panahi A, Khazaeipour Z, Barari F, Sahebnasagh A, et al. Negative correlation between serum S100B and leptin levels in schizophrenic patients during treatment with clozapine and risperidone: preliminary evidence. Iran J Pharm Res 2016;15:323-330.


77. Lara DR, Gama CS, Belmonte-de-Abreu P, Portela LV, Gonçalves CA, Fonseca M, et al. Increased serum S100B protein in schizophrenia: a study in medication-free patients. J Psychiatr Res 2001;35:11-14.


78. Ling SH, Tang YL, Jiang F, Wiste A, Guo SS, Weng YZ, et al. Plasma S-100B protein in Chinese patients with schizophrenia: comparison with healthy controls and effect of antipsychotics treatment. J Psychiatr Res 2007;41:36-42.


80. Morera-Fumero AL, Díaz-Mesa E, Abreu-Gonzalez P, Fernandez-Lopez L, Cejas-Mendez MDR. Day/night changes in serum S100B protein concentrations in acute paranoid schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2017;75:207-212.


81. Qi LY, Xiu MH, Chen DC, Wang F, Kosten TA, Kosten TR, et al. Increased serum S100B levels in chronic schizophrenic patients on long-term clozapine or typical antipsychotics. Neurosci Lett 2009;462:113-117.


85. Rothermundt M, Ohrmann P, Abel S, Siegmund A, Pedersen A, Ponath G, et al. Glial cell activation in a subgroup of patients with schizophrenia indicated by increased S100B serum concentrations and elevated myo-inositol. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:361-364.


86. Kim HR, Lee MK, Park DB. Increased serum S100B protein in chronic schizophrenic patients in Korea. Clin Chem Lab Med 2007;45:1561-1563.

87. Šakić M, Karlović D, Vidrih B, Peitl V, Crnković D, Vrkić N. Increased calcium-independent lipoprotein phospholipase A2 but not protein S100 in patients with schizophrenia. Psychiatr Danub 2016;28:45-50.
88. Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E. Oxidative-antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:1164-1169.


89. Schmitt A, Bertsch T, Henning U, Tost H, Klimke A, Henn FA, et al. Increased serum S100B in elderly, chronic schizophrenic patients: negative correlation with deficit symptoms. Schizophr Res 2005;80:305-313.


90. Schroeter ML, Abdul-Khaliq H, Frühauf S, Höhne R, Schick G, Diefenbacher A, et al. Serum S100B is increased during early treatment with antipsychotics and in deficit schizophrenia. Schizophr Res 2003;62:231-236.


93. Uzbay T, Goktalay G, Kayir H, Eker SS, Sarandol A, Oral S, et al. Increased plasma agmatine levels in patients with schizophrenia. J Psychiatr Res 2013;47:1054-1060.


94. Wiesmann M, Wandinger KP, Missler U, Eckhoff D, Rothermundt M, Arolt V, et al. Elevated plasma levels of S-100b protein in schizophrenic patients. Biol Psychiatry 1999;45:1508-1511.


95. Xiong P, Zeng Y, Wu Q, Han Huang DX, Zainal H, Xu X, et al. Combining serum protein concentrations to diagnose schizophrenia: a preliminary exploration. J Clin Psychiatry 2014;75:e794-801.


96. Zhang XY, Xiu MH, Song C, Chen DC, Wu GY, Haile CN, et al. Increased serum S100B in never-medicated and medicated schizophrenic patients. J Psychiatr Res 2010;44:1236-1240.


97. Gattaz WF, Lara DR, Elkis H, Portela LV, Gonçalves CA, Tort AB, et al. Decreased S100-beta protein in schizophrenia: preliminary evidence. Schizophr Res 2000;43:91-95.


98. O’Connell K, Thakore J, Dev KK. Levels of S100B are raised in female patients with schizophrenia. BMC Psychiatry 2013;13:146