1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789-1858.


2. Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep 2019;21:10


3. Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. Global Burden of Mental, Neurological, and Substance Use Disorders: An Analysis from the Global Burden of Disease Study 2010 (3rd Ed). In: Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME, editor. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities. Washington, DC: The International Bank for Reconstruction and Development/The World Bank, 2016.
4. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem 2006;97:1634-1658.


5. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008;11:851-876.


6. Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegaliński E, et al. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep 2015;67:569-580.


7. Bulbul F, Virit O, Alpak G, Unal A, Bulut M, Kaya MC, et al. Are oxidative stress markers useful to distinguish schizoaffective disorder from schizophrenia and bipolar disorder? Acta Neuropsychiatr 2014;26:120-124.


8. Zhang M, Zhao ZM, He L, Wan CL. A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci 2010;53:112-124.


10. Hickerson RP, Prat F, Muller JG, Foote CS, Burrows CJ. Sequence and stacking dependence of 8-oxoguanine oxidation: comparison of oneelectron vs singlet oxygen mechanisms. J Am Chem Soc 1999;121:9423-9428.

12. Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2’-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:120-139.


14. Korkmaz KS, Butuner BD, Roggenbuck D. Detection of 8-OHdG as a diagnostic biomarker. J Lab Precis Med 2018;3:95

16. Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res 2014;218:61-68.


18. Christensen MR, Poulsen HE, Henriksen T, Weimann A, Ellervik C, Lynnerup N, et al. Elevated levels of 8-oxoGuo and 8-oxodG in individuals with severe mental illness - An autopsy-based study. Free Radic Biol Med 2018;126:372-378.


19. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I
2 Index? Psychol Methods 2006;11:193-206.

20. Lin E, Tong T, Chen Y, Wang Y. Fixed-effects model: the most convincing model for meta-analysis with few studies. Preprint arXiv 2002.04211
22. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-463.


24. Ershova ES, Jestkova EM, Chestkov IV, Porokhovnik LN, Izevskaya VL, Kutsev SI, et al. Quantification of cell-free DNA in blood plasma and DNA damage degree in lymphocytes to evaluate dysregulation of apoptosis in schizophrenia patients. J Psychiatr Res 2017;87:15-22.


25. Shmarina GV, Orlova MD, Ershova ES, Jestkova EM, Martynov AV, Veiko NN, et al. NRF2 and HMOX1 gene expression against the background of systemic oxidative stress in patients with acute psychosis. Russ J Genet 2020;56:96-102.

26. Jorgensen A, Broedbaek K, Fink-Jensen A, Knorr U, Soendergaard MG, Henriksen T, et al. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res 2013;209:417-423.


27. Nordholm D, Poulsen HE, Hjorthøj C, Randers L, Nielsen MØ, Wulff S, et al. Systemic oxidative DNA and RNA damage are not increased during early phases of psychosis: a case control study. Psychiatry Res 2016;241:201?206


28. Ibrahim RR, Amer RA, Abozeid AA, Elsharaby RM, Shafik NM. Micro RNA 146a gene variant/TNF-α/IL-6/IL-1 β; a cross-link axis in between oxidative stress, endothelial dysfunction and neuro-inflammation in acute ischemic stroke and chronic schizophrenic patients. Arch Biochem 2020;679:108193


29. Ma J, Yan L, Guo T, Yang S, Ni D, Liu Y, et al. A pilot study of biomarkers of oxidative stress in serum and schizophrenia. Psychiatry Res 2020;284:112757


30. Copoglu US, Virit O, Kokacya MH, Orkmez M, Bulbul F, Erbagci AB, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res 2015;229:200-205.


31. Şimşek Ş, Gençoğlan S, Yüksel T, Kaplan İ, Alaca R, Aktaş H. Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology 2016;73:92-97.


32. Miyaoka T, Ieda M, Hashioka S, Wake R, Furuya M, Liaury K, et al. Analysis of oxidative stress expressed by urinary level of biopyrrins and 8-hydroxydeoxyguanosine in patients with chronic schizophrenia. Psychiatry Clin Neurosci 2015;69:693-698.


34. Munkholm K, Poulsen HE, Kessing LV, Vinberg M. Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder. Bipolar Disord 2015;17:257-268.


36. Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol 2013;16:1505-1512.


37. Tsai MC, Huang TL. Thiobarbituric acid reactive substances (TBARS) is a state biomarker of oxidative stress in bipolar patients in a manic phase. J Affect Disord 2015;173:22-26.


38. Huzayyin AA, Andreazza AC, Turecki G, Cruceanu C, Rouleau GA, Alda M, et al. Decreased global methylation in patients with bipolar disorder who respond to lithium. Int J Neuropsychopharmacol 2014;17:561-569.


39. Ceylan D, Yılmaz S, Tuna G, Kant M, Er A, Ildız A, et al. Alterations in levels of 8-Oxo-2’-deoxyguanosine and 8-Oxoguanine DNA glycosylase 1 during a current episode and after remission in unipolar and bipolar depression. Psychoneuroendocrinology 2020;114:104600


40. Yi S, Nanri A, Matsushita Y, Kasai H, Kawai K, Mizoue T. Depressive symptoms and oxidative DNA damage in Japanese municipal employees. Psychiatry Res 2012;200:318-322.


41. Black CN, Bot M, Scheffer PG, Penninx BWJH. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol Med 2017;47:936-948.


42. van Velzen LS, Wijdeveld M, Black CN, van Tol MJ, van der Wee NJA, Veltman DJ, et al. Oxidative stress and brain morphology in individuals with depression, anxiety and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry 2017;76:140-144.


43. Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017;76:197-205.


44. Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom Med 2006;68:1-7.


45. Tsai MC, Huang TL. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res 2016;235:38-42.


46. Wei Y, Zhou F, He D, Bai J, Hui L, Wang X, et al. The level of oxidative stress and the expression of genes involved in DNA-damage signaling pathways in depressive patients with colorectal carcinoma. J Psychosom Res 2009;66:259-266.


47. Wei YC, Zhou FL, He DL, Bai JR, Ding H, Wang XY, et al. Oxidative stress in depressive patients with gastric adenocarcinoma. Int J Neuropsychopharmacol 2009;12:1089-1096.


49. Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets 2005;16:386-391.


50. Dietrich-Muszalska A, Olas B. Modifications of blood platelet proteins of patients with schizophrenia. Platelets 2009;20:90-96.


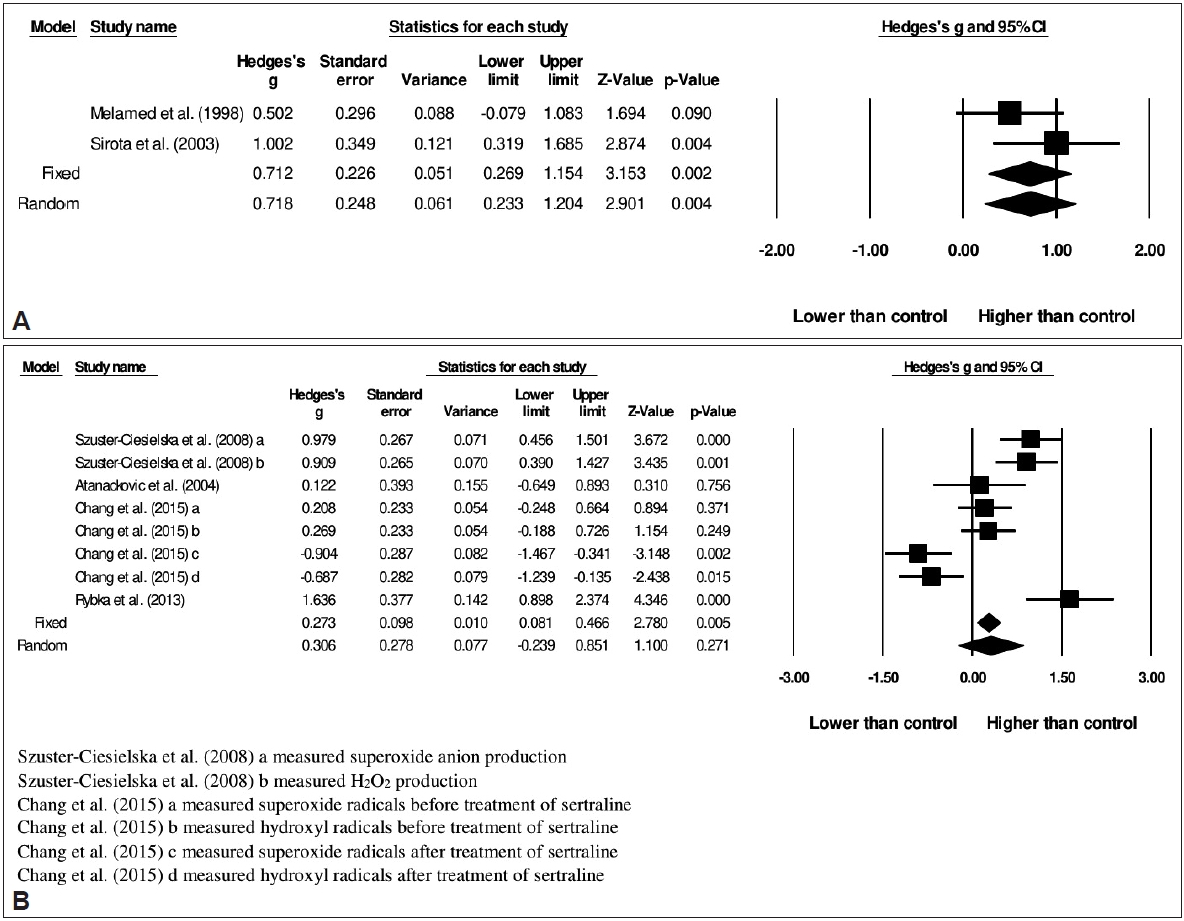
51. Melamed Y, Sirota P, Dicker DR, Fishman P. Superoxide anion production by neutrophils derived from peripheral blood of schizophrenic patients. Psychiatry Res 1998;77:29-34.


52. Sirota P, Gavrieli R, Wolach B. Overproduction of neutrophil radical oxygen species correlates with negative symptoms in schizophrenic patients: parallel studies on neutrophil chemotaxis, superoxide production and bactericidal activity. Psychiatry Res 2003;121:123-132.


53. Szuster-Ciesielska A, Słotwińska M, Stachura A, MarmurowskaMichałowska H, Dubas-Ślemp H, Bojarska-Junak A, et al. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:686-694.


54. Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun 2014;36:111-117.


55. Atanackovic D, Kröger H, Serke S, Deter HC. Immune parameters in patients with anxiety or depression during psychotherapy. J Affect Disord 2004;81:201-209.


56. Chang CC, Lee CT, Lan TH, Ju PC, Hsieh YH, Lai TJ. Effects of antidepressant treatment on total antioxidant capacity and free radical levels in patients with major depressive disorder. Psychiatry Res 2015;230:575-580.


57. Rybka J, Kędziora-Kornatowska K, Banaś-Leżańska P, Majsterek I, Carvalho LA, Cattaneo A, et al. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med 2013;63:187-194.


58. Andreazza AC, Kauer-Sant’Anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a metaanalysis. J Affect Disord 2008;111:135-144.


62. Muraleedharan A, Menon V, Rajkumar RP, Chand P. Assessment of DNA damage and repair efficiency in drug naïve schizophrenia using comet assay. J Psychiatr Res 2015;68:47-53.


63. Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry 2004;12:167-175.


64. Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ 2012;345:e8508


65. Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, et al. DNA damage in bipolar disorder. Psychiatry Res 2007;153:27-32.


66. Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, et al. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry 2007;31:283-285.


68. Mustak MS, Hegde ML, Dinesh A, Britton GB, Berrocal R, Rao KS, et al. Evidence of altered DNA integrity in the brain regions of suicidal victims of bipolar depression. Indian J Psychol Med 2010;52:220-228.

69. Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LMV, Nardin P, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res 2007;41:523-529.


70. Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol 1997;48:191-214.


72. Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment. J Clin Psychiatry 2015;76:1658-1667.


73. Palta P, Samuel LJ, Miller ER, Szanton SL. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom Med 2014;76:12-19.

74. Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BWJH. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015;51:164-175.


75. Fletcher J. What is heterogeneity and is it important? Br Med J 2007;334:94-96.

76. Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 2000;53:207-216.


77. Mlinarić A, Horvat M, Smolčić VŠ. Dealing with the positive publication bias: why you should really publish your negative results. Biochem Med 2017;27:447-452.

78. Henriksen T, Hillestrøm PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2’-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med 2009;47:629-635.


79. Poulsen HE, Nadal LL, Broedbaek K, Nielsen PE, Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim Biophys Acta 2014;1840:801-808.


80. Collins AR, Oscoz AA, Brunborg G, Gaivão I, Giovannelli L, Kruszewski M, et al. The comet assay: topical issues. Mutagenesis 2008;23:143-151.


81. Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr 2000;72:1082-1087.


82. Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev 2008;32:675-692.


83. Kapczinski F, Dias VV, Kauer-Sant’Anna M, Brietzke E, Vázquez GH, Vieta E, et al. The potential use of biomarkers as an adjunctive tool for staging bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:1366-1371.


84. Nguyen TT, Eyler LT, Jeste DV. Systemic biomarkers of accelerated aging in schizophrenia: a critical review and future directions. Schizophr Bull 2018;44:398-408.


85. Abdel-Wahab BA, Abdalla ME, El-khawanki MM. Does clozapine induce myocarditis, myocardial oxidative stress and DNA damage in rats? Egypt J Forensic Sci 2014;4:75-82.

87. Williams RE, Lock EA. Sodium benzoate attenuates D-serine induced nephrotoxicity in the rat. Toxicology 2005;207:35-48.


88. Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry 2018;84:422-432.


89. Shishikura M, Hakariya H, Iwasa S, Yoshio T, Ichiba H, Yorita K, et al. Evaluation of human D-amino acid oxidase inhibition by anti-psychotic drugs in vitro. Biosci Trends 2014;8:149-154.


90. Martins MR, Petronilho FC, Gomes KM, Dal-Pizzol F, Streck EL, Quevedo J. Antipsychotic-induced oxidative stress in rat brain. Neurotox Res 2008;13:63-69.


91. Freese E, Sklarow S, Freese EB. DNA damage caused by antidepressant hydrazines and related drugs. Mutat Res 1968;5:343-348.


92. Alosaimi FD, Alhabbad A, Abalhassan MF, Fallata EO, Alzain NM, Alassiry MZ, et al. Patterns of psychotropic medication use in inpatient and outpatient psychiatric settings in Saudi Arabia. Neuropsychiatr Dis Treat 2016;12:897-907.


94. Post A, Crochemore C, Uhr M, Holsboer F, Behl C. Differential induction of NF‐κB activity and neural cell death by antidepressants in vitro. Eur J Neurosci 2000;12:4331-4337.