|
 |
- Search
| Psychiatry Investig > Volume 17(8); 2020 > Article |
|
Abstract
Objective
Methods
Results
ACKNOWLEDGEMENTS
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Minyoung Shin, Ahee Lee, Yun-Hee Kim. Data curation: Ahee Lee, A Young Cho, Minam Son. Formal analysis: Minyoung Shin. Funding acquisition: Yun-Hee Kim. Investigation: Minyoung Shin, Ahee Lee. Methodology: Minyoung Shin, Ahee Lee, A Young Cho, Minam Son. Project administration: Yun-Hee Kim. Resources: Yun-Hee Kim. Software: Minyoung Shin, Ahee Lee, Yun-Hee Kim. Supervision: Yun-Hee Kim. Validation: Minyoung Shin, Ahee Lee, Yun-Hee Kim. Visualization: Minyoung Shin. WritingŌĆöoriginal draft: Minyoung Shin. WritingŌĆöreview & editing: Yun-Hee Kim.
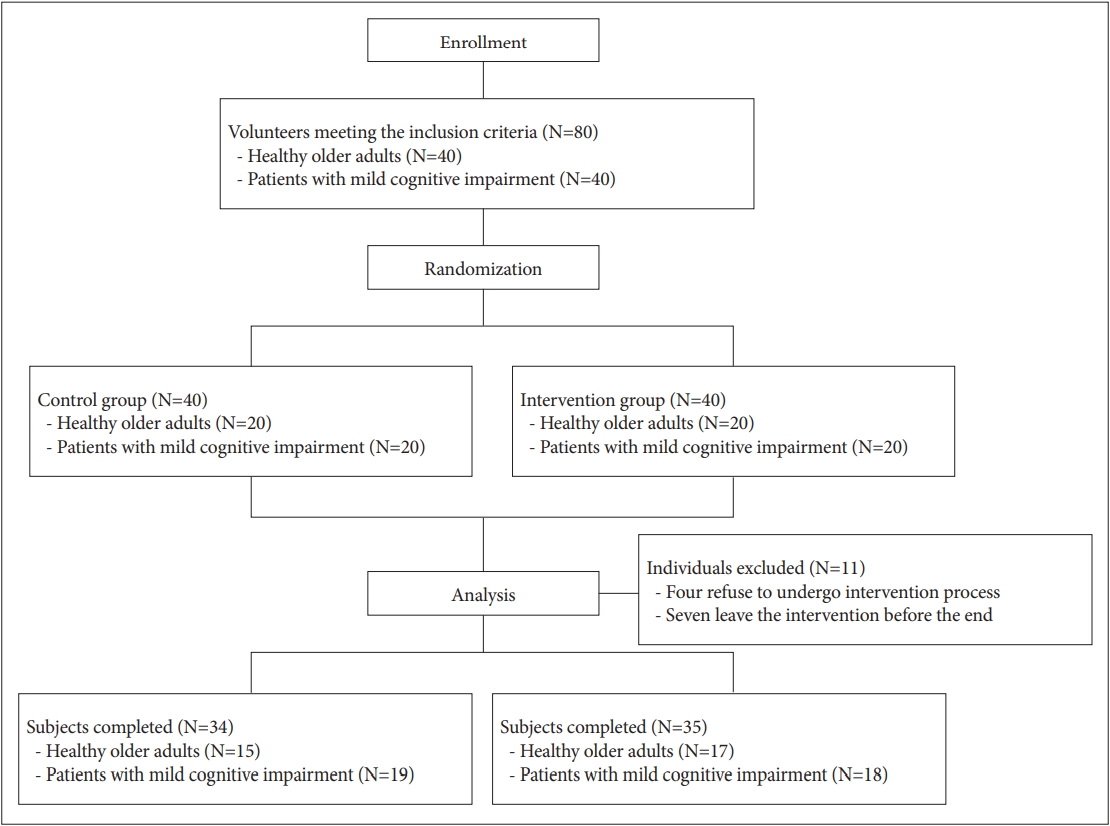
Figure┬Ā2.
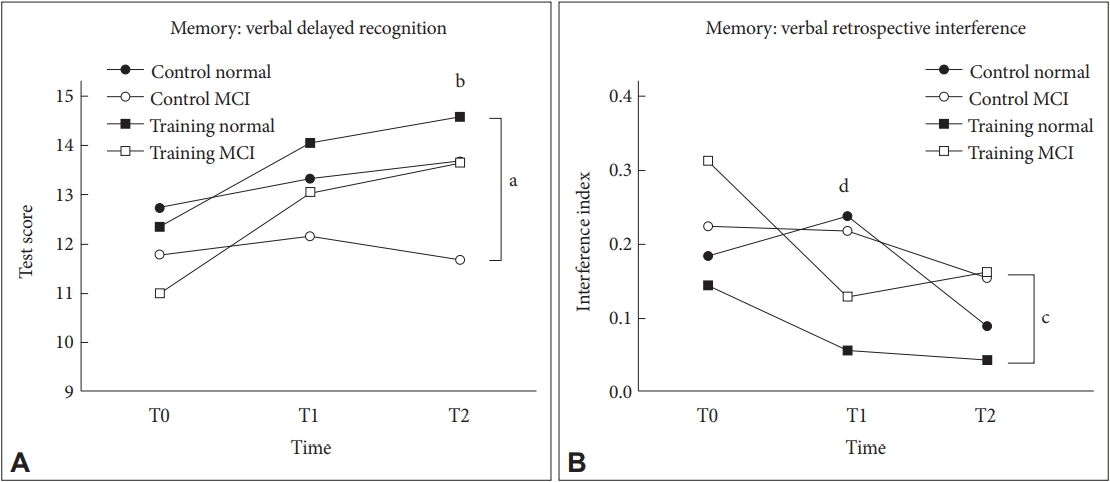
Figure┬Ā3.

Table┬Ā1.
|
Control group (N=34) |
Experimental (training) group (N=35) |
t/Žć2 | |||||
|---|---|---|---|---|---|---|---|
| All (N=34) | Healthy (N=15) | MCI (N=19) | All (N=35) | Healthy (N=17) | MCI (N=18) | ||
| Age, M (SD) | 65.44 (8.10) | 61.33 (6.49) | 68.68 (7.90) | 65.31 (7.47) | 62.00 (6.50) | 68.44 (7.11) | 0.068 |
| Education, M (SD) | 11.71 (3.72) | 12.60 (2.53) | 11.00 (4.38)* | 13.26 (3.47) | 12.71 (3.33) | 13.78 (3.61)* | -1.792 |
| Sex, M:F | 8:26 | 4:11 | 4:15 | 9:26 | 3:14 | 6:12 | 0.044 |
Table┬Ā2.
| Measures |
Control group |
Experimental (training) group |
RMANOVA |
ANCOVA |
|||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | F | F (T1) | F (T2) | |
| Global cognitive function | |||||||||
| ŌĆāMoCA-K | 23.26┬▒2.59 | 24.65┬▒3.30 | 24.68┬▒2.87 | 23.97┬▒3.48 | 25.06┬▒2.87 | 26.37┬▒3.48 | 3.35* | 0.22 | 5.21* |
| Attention | |||||||||
| ŌĆāDigit span forwardŌĆĀ | 5.56┬▒1.65 | 5.76┬▒1.48 | 5.91┬▒1.22 | 5.71┬▒1.24 | 6.38┬▒1.16 | 6.65┬▒1.04 | 4.60* | 3.68 | 11.77** |
| ŌĆāDigit span backwardŌĆĀ | 4.35┬▒1.20 | 4.21┬▒0.95 | 4.35┬▒1.39 | 4.38┬▒1.33 | 4.76┬▒1.26 | 4.97┬▒1.24 | 4.68* | 8.02** | 2.61 |
| ŌĆāVisual span forwardŌĆĀ | 5.12┬▒1.37 | 5.38┬▒1.02 | 5.71┬▒0.91 | 5.50┬▒1.05 | 5.65┬▒0.85 | 5.62┬▒0.95 | 1.06 | 0.40 | 0.58 |
| ŌĆāVisual span backward | 5.26┬▒0.99 | 5.35┬▒1.04 | 5.24┬▒1.05 | 4.85┬▒1.18 | 5.03┬▒1.03 | 5.32┬▒0.73 | 1.98 | 2.12 | 0.29 |
| ŌĆāACPT_rtŌĆĀ | 0.69┬▒0.06 | 0.68┬▒0.05 | 0.67┬▒0.05 | 0.68┬▒0.07 | 0.67┬▒0.07 | 0.67┬▒0.09 | 1.08 | 0.26 | 0.91 |
| ŌĆāVCPT_rtŌĆĀ | 0.45┬▒0.06 | 0.45┬▒0.05 | 0.45┬▒0.05 | 0.44┬▒0.06 | 0.44┬▒0.06 | 0.44┬▒0.07 | 0.01 | 0.01 | 0.03 |
| Memory | |||||||||
| ŌĆāLearn total | 43.09┬▒8.91 | 50.76┬▒14.13 | 56.15┬▒14.11 | 46.85┬▒9.66 | 54.85┬▒11.64 | 60.85┬▒11.59 | 0.20 | 0.01 | 0.48 |
| ŌĆāLearn index | 5.74┬▒2.37 | 5.62┬▒2.19 | 3.79┬▒3.49 | 6.29┬▒2.21 | 5.59┬▒2.56 | 3.74┬▒2.39 | 0.58 | 0.06 | 3.17 |
| ŌĆāDelayed recallŌĆĀ | 9.06┬▒2.71 | 10.35┬▒3.28 | 11.41┬▒3.48 | 9.76┬▒3.24 | 11.53┬▒3.26 | 12.71┬▒3.19 | 0.50 | 0.42 | 1.82 |
| ŌĆāDelayed recognitionŌĆĀ | 12.21┬▒2.16 | 12.68┬▒2.66 | 12.56┬▒3.32 | 11.68┬▒3.91 | 13.56┬▒1.65 | 14.12┬▒1.65 | 4.02* | 4.14* | 6.70* |
| ŌĆāPercentage of forgetting | 0.19┬▒0.12 | 0.18┬▒0.17 | 0.12┬▒0.18 | 0.19┬▒0.20 | 0.13┬▒0.17 | 0.09┬▒0.20 | 0.63 | 1.89 | 0.84 |
| ŌĆāProactive interference | -0.01┬▒0.45 | 0.04┬▒0.74 | 0.38┬▒0.35 | -0.07┬▒0.44 | 0.14┬▒0.37 | 0.38┬▒0.30 | 0.63 | 0.75 | 1.89 |
| ŌĆāRetroactive interferenceŌĆĀ | 0.21┬▒0.17 | 0.23┬▒0.18 | 0.12┬▒0.17 | 0.23┬▒0.24 | 0.09┬▒0.14 | 0.10┬▒0.18 | 7.04** | 14.04*** | 0.91 |
| Executive function | |||||||||
| ŌĆāStroop interferenceŌĆĀ | 2.10┬▒0.77 | 1.92┬▒0.62 | 1.79┬▒0.41 | 2.15┬▒0.61 | 1.94┬▒0.56 | 1.84┬▒0.56 | 0.09 | 0.04 | 0.10 |
| ŌĆāTMT B_rt | 85.53┬▒43.14 | 77.44┬▒39.70 | 73.97┬▒40.97 | 86.35┬▒29.74 | 80.18┬▒25.51 | 72.88┬▒33.00 | 0.12 | 1.92 | 0.54 |
| Depression | |||||||||
| ŌĆāK-GDS-SF | 4.44┬▒3.48 | 4.18┬▒2.79 | 5.18┬▒4.01 | 4.49┬▒3.67 | 3.86┬▒3.01 | 3.77┬▒3.73 | 3.58* | 0.27 | 7.42** |
RMANOVA was carried out with age, sex, and education as covariates. ANCOVA was carried out with age, sex, education, and test score at T0 as covariates.
T0, pre-intervention; T1, post-intervention; T2, four-week follow-up. MoCA-K: Korean Version of the Montreal Cognitive Assessment, ACPT_rt: Auditory Continuous Performance Test_reaction time, VCPT_rt: Visual Continuous Performance Test_reaction time, TMT B_rt: Trail Making Test B_ reaction time, K-GDS-SF: Korean Version of the Geriatric Depression Scale Short Form, RMANOVA: repeated measure analysis of variance, ANCOVA: analysis of covariance
Table┬Ā3.
| Measures |
RMANOVA |
ANCOVA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Df |
F |
Df |
F |
||||||||
| Time├Śtraining | Time├ŚMCI | Time├Śtraining├ŚMCI |
Post intervention |
Four weeks after intervention |
|||||||
| Training | MCI | Training├ŚMCI | Training | MCI | Training├ŚMCI | ||||||
| Global cognitive function | |||||||||||
| ŌĆāMoCA-K | 2 | 3.23* | 0.06 | 0.24 | 1 | 0.18 | 0.14 | 2.11 | 5.13*** | 0.00 | 0.24 |
| Attention | |||||||||||
| ŌĆāDigit span forwardŌĆĀ | 2 | 4.53* | 0.34 | 1.64 | 1 | 3.51 | 0.54 | 1.44 | 11.29* | 0.83 | 0.00 |
| ŌĆāDigit span backwardŌĆĀ | 2 | 4.60* | 0.09 | 0.84 | 1 | 7.83** | 0.20 | 0.52 | 2.47 | 0.03 | 0.17 |
| ŌĆāVisual span forwardŌĆĀ | 2 | 1.37 | 1.22 | 2.72 | 1 | 0.37 | 0.37 | 0.51 | 0.58 | 0.10 | 0.00 |
| ŌĆāVisual span backward | 2 | 0.02 | 2.27 | 1.28 | 1 | 2.01 | 0.28 | 0.84 | 0.16 | 0.06 | 0.70 |
| ŌĆāACPT_rt | 2 | 0.95 | 0.74 | 0.40 | 1 | 0.05 | 0.34 | 1.35 | 0.25 | 0.05 | 0.80 |
| ŌĆāVCPT_rt | 2 | 1.87 | 0.61 | 0 .18 | 1 | 0.02 | 0.97 | 0.56 | 0.05 | 2.92 | 0.92 |
| Memory | |||||||||||
| ŌĆāLearn total | 2 | 0.22 | 0.78 | 0.17 | 1 | 0.01 | 0.68 | 0.20 | 0.47 | 0.13 | 0.11 |
| ŌĆāLearn index | 2 | 0.55 | 0.66 | 1.07 | 1 | 0.04 | 2.09 | 1.74 | 0.12 | 0.04 | 0.31 |
| ŌĆāDelayed recall | 2 | 0.50 | 0.01 | 0.09 | 1 | 0.40 | 0.01 | 0.04 | 1.75 | 0.00 | 0.01 |
| ŌĆāDelayed recognitionŌĆĀ | 2 | 4.02* | 0.05 | 0.64 | 1 | 3.99 | 0.01 | 0.01 | 6.53* | 0.02 | 0.26 |
| ŌĆāPercentage of forgetting | 2 | 0.62 | 0.65 | 1.68 | 1 | 1.93 | 0.23 | 5.08* | 0.77 | 0.48 | 0.00 |
| ŌĆāProactive interference | 2 | 0.64 | 0.01 | 0.75 | 1 | 0.74 | 0.01 | 0.84 | 0.22 | 0.08 | 2.84 |
| ŌĆāRetroactive interferenceŌĆĀ | 2 | 7.26** | 1.20 | 0.40 | 1 | 14.09*** | 0.71 | 0.75 | 0.88 | 0.01 | 0.08 |
| Executive function | |||||||||||
| ŌĆāStroop interference | 2 | 0.08 | 3.05 | 0 .01 | 1 | 0.07 | 2.59 | 0.74 | 0.16 | 5.13* | 0.91 |
| ŌĆāTMT B_rt | 2 | 0.11 | 0.29 | 0.10 | 1 | 1.84 | 0.33 | 0.90 | 0.54 | 0.06 | 0.20 |
| Depression | |||||||||||
| ŌĆāK_GDS_SF | 2 | 3.66* | 0.56 | 0.56 | 1 | 0.29 | 0.05 | 0.77 | 7.68** | 0.58 | 1.07 |
RMANOVA was carried out with age, sex, and education as covariates. ANCOVA was carried out with age, sex, education, and test score at pre-intervention as covariates.
MoCA-K: Korean Version of the Montreal Cognitive Assessment, ACPT_rt: Auditory Continuous Performance Test_reaction time, VCPT_rt: Visual Continuous Performance Test_reaction time, TMT B_rt: Trail Making Test B_ reaction time, K-GDS-SF: Korean Version of the Geriatric Depression Scale Short Form, RMANOVA: repeated measure analysis of variance, ANCOVA: analysis of covariance
REFERENCES