Pharmacological and Neuromodulatory Treatments for Panic Disorder: Clinical Trials from 2010 to 2018
Article information
Abstract
Objective
Treatment for panic disorder (PD) have evolved, although there is still a strong unmet need for more effective and tolerable options. The present study summarizes and discusses recent evidence regarding the pharmacological and neuromodulatory treatment of PD.
Methods
MEDLINE, Cochrane Library, PsycINFO and Thomson Reuters’s Web of Science were searched for clinical trials published between 2010 and 2018. We included all prospective experimental studies including randomized controlled trials (RCT) and other clinical trials with more than 10 patients.
Results
Only 11 articles met the inclusion criteria, including 4 RCT, 3 open clinical trials and 5 comparative clinical trials. RCT demonstrated efficacy of transcranial magnetic stimulation (TMS) in only one of two trials. Neither pindolol nor d-fenfluramine were effective in blocking flumazenil-induced panic attacks. Augmentation with quetiapine was not superior to placebo. Open trials indicated that escitalopram, vortioxetine and TMS may be effective. Comparative trials did not demonstrate superiority from any drug, but confirmed tranylcypromine, paroxetine, clonazepam and alprazolam as effective options.
Conclusion
The current study confirmed the efficacy of tranylcypromine, paroxetine, clonazepam, alprazolam and escitalopram. Vortioxetine and TMS, with duration of 4 or more weeks, also seems to be effective. Quetiapine, pindolol and d-fenfluramine were not considered effective compounds.
INTRODUCTION
Panic disorder (PD) is defined by recurrent, unexpected panic attacks (PA), wherein at least one PA must be followed by at least one month of persistent concern about having more attacks, worry about the consequences of the attacks, or maladaptive behavior related to the attacks [1]. PD is common in the general population with a lifetime prevalence of 1.6% to 2.2% [2] and is associated with high rate of relapse, psychiatric/medical comorbidity, significant impairment of quality of life and relevant social costs [3].
Pharmacological treatment of panic disorder emerged in 1959, when Donald F. Klein established the beneficial effects of the tricyclic antidepressant imipramine [4]. The selective serotonin reuptake inhibitors (SSRI) have been used in the treatment of patients with panic disorder since the 1980s, followed by the dual reuptake inhibitor venlafaxine in the subsequent decade [4]. Several medications have been used effectively in the treatment of PD, including SSRI, serotonin-norepinephrine reuptake inhibitors (SNRI), tricyclic antidepressants (TCA) and benzodiazepines (BDZ), however, approximately 20% to 40% of the subjects with PD do not fully respond to pharmacotherapy [3,5]. A similar rate does not improve with cognitive behavioral therapy (CBT), and so far, combining CBT to pharmacotherapy has not sufficiently filled this gap [3]. In addition, 25% to 50% of patients relapse within 6 months after drug discontinuation and up to 50% still experience residual panic phobic symptoms [6]. Finally, up to 30% of patients still suffer from a full-blown disorder after 3 to 6 years [6].
From a clinical perspective, there is still a strong unmet need for effective, fast acting and tolerable therapeutic treatments for PD [3]. Many reasons may explain the difficulties to fill these gaps [3]. First, PD is a heterogeneous condition that results from the interplay of unexpected PAs, and other symptoms, that is, anticipatory anxiety and phobic behaviors associated with expected PAs [3]. Second, the underlying pathophysiology of PD is still under study, not entirely clear. Some contemporary theories conceive PAs as primal defensive reactions to threat within the internal milieu of the body, which might be attributable to a misfiring suffocation alarm and/or malfunction of brain circuits modulating defensive responses [3]. Third, several neurotransmitters acting in different central nervous system (CNS) areas and influencing each other may be involved in modulating these putative processes [3].
The serotonergic system plays a relevant role in regions of the brain involved both in control of ventilation and acid-base balance and in emotional responses, arousal and defensive behaviors, including brainstem respiratory network, the nucleus tractus solitarii, the medullary and midbrain raphe neurons, the amygdala and the hypothalamus, both having CO2/H+ sensitive neurons, and the periaqueductal gray [6]. Serotonergic system may have an inhibitory action on locus coeruleus and amygdala and reduces hypothalamic release of corticotrophin-releasing factor (CRF), thus modulating behavioral and physiological responses to fear or stressful stimuli. Neurons in the noradrenergic locus coeruleus are CO2/H+ sensitive, most likely serving both respiration and defensive responses, and their firing is enhanced by CO2 inhalation. Noradrenergic agents diminish reactivity to CO2 inhalation in patients with PD, even though the decrease is significantly weaker than in patients treated with serotonergic agents. Noradrenergic medications may blunt the phasic noradrenergic reactivity to threatening stimuli and stressful situations, reducing autonomic arousal and behavioral activation [6].
Similarly, the γ-aminobutyric-acid GABA system influences activity of several sites involved in autonomic, respiratory and behavioral responses, including brainstem, hypothalamus and limbic structures [6]. Increased activity in the emotion-processing brain regions could result from decreased inhibitory signaling by GABA or increased excitatory neurotransmission by glutamate, in patients with an anxiety disorder [7]. Benzodiazepines and anticonvulsant drugs may have antipanic effects through reduction of neuronal excitability in the limbic structures, mediated by the GABA-A receptors [7]. Studies have demonstrated that benzodiazepines are effective in the blockade of CO2-induced PAs as well as in the clinical treatment of PD [7,8], whereas the effectiveness of anticonvulsant drugs in the literature sparse and still under discussion [7,9].
On a neurobiological level, functional imaging studies of PD patients with and without agoraphobia have found hypoactivity of the prefrontal cortex (PFC), paired with hyperactivity of fear relevant brain structures such as the amygdala, suggesting an inadequate inhibition by the PFC in response to anxiety-related stimuli [10]. Cortical activation patterns can be selectively modified by means of repetitive transcranial magnetic stimulation (rTMS) via electromagnetic induction [10]. rTMS uses powerful but extremely brief magnetic fields which induce a current in the brain. It has been applied for evaluation of the motor system, functional research of cerebral regions, and pathophysiological mechanisms of mental disorders [11]. TMS has also developed as an intervention tool, which is administered in a rhythmic and repetitive form and is thus referred to as repetitive transcranial magnetic stimulation (rTMS) [11]. In rTMS, trains of magnetic pulses temporarily summate to cause greater changes in neural activity than a single pulse, which can modulate cortical excitability. While highfrequency rTMS is considered to increase the cortical excitability in certain regions, low-frequency rTMS is postulated to inhibit the cortical excitability of stimulated area [11]. This way, rTMS has been shown to modulate neurotransmitter release and - depending on its stimulation frequency - normalize prefrontal hypoactivity [10].
In a previous article [7], the authors reviewed the pharmacological trials for PD from 2000 to 2010 and found that the effectiveness of SSRI, monoamine oxidase inhibitors (MAOI), tricyclic antidepressants, venlafaxine and benzodiazepines was well stablished, while there was preliminary evidence demonstrating efficacy for duloxetine, nefazodone, mirtazapine, inositol and milnacipram. Pindolol and clonazepam were effective in augmentation strategies. We expected that in the last 8 years the effectiveness of older drugs would be confirmed and new drugs would prove to be effective in the treatment of PD.
The aim of this study was to summarize and discuss recent evidence regarding the pharmacological and neuromodulatory treatment of PD, based on clinical trials available from 2010 to 2018.
METHODS
Articles were identified by a search of electronic records, including the databases from MEDLINE/Pubmed, the Cochrane Library, PsycINFO and Thomson Reuters’s Web of Science. The search terms used were: “panic disorder” AND (“treatment/treatments” OR “therapy/therapies” OR “effect/effects” OR “clinical trial” OR “randomized controlled trial”). Only studies published between 2010 and 2018, in English, with human subjects, considered “journal articles” and clinical trial were included. We included trials recruiting only adult subjects with PD, consistent with criteria from DSM-IV to DSM-5. We included all prospective experimental studies including randomized controlled trials (RCT), quasi-random trials, crossover designs, and single arm studies, blinded or open label. Case, case series, retrospective studies or studies with less than ten PD subjects were not included. Studies that reported selectively on specific subpopulations based on age or gender were not included. Filters with this criteria were applied in the databases were it was possible.
In the first step of the process, the first author (MMZ) screened the titles and abstracts of the articles and manually excluded those that did not fit the criteria mentioned above. The second step was the study selection. Two independent reviewers (MMZ and MCC) assessed the full-text articles for eligibility; this assessment was made in a standardized manner. The same reviewers made the data extraction in an independent manner. Disagreements between the reviewers regarding the study selection or data extraction were resolved by consensus.
Since we included trials with different designs, the presence of a controlling condition, randomization and subject and observer blindness to treatment were used as measures of strength of evidence.
The ideal confirmatory format of this review would consist of meta-analyses of interaction statistics of predictors and moderators effects of large, high quality, RCTs. Since most trials did not meet quality requirements, and a broad spectrum of experimental designs was included, we performed a qualitative systematic review of the evidence.
RESULTS
The searches of databases were conducted in May of 2018 and yielded 1,680 articles: 384 in Cochrane Library, 512 articles in PsycINFO, 264 in MEDLINE/Pubmed and 520 in Web of Science. The sum of articles after removing the duplicates was 925, and only 86 articles remained after the title and abstract. Reviewers examined the full-texts and only 11 articles met the inclusion criteria. The inter-rater agreement was substantial, with a free marginal kappa coefficient of 0.74. The process of study identification and selection is shown in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Figure 1).
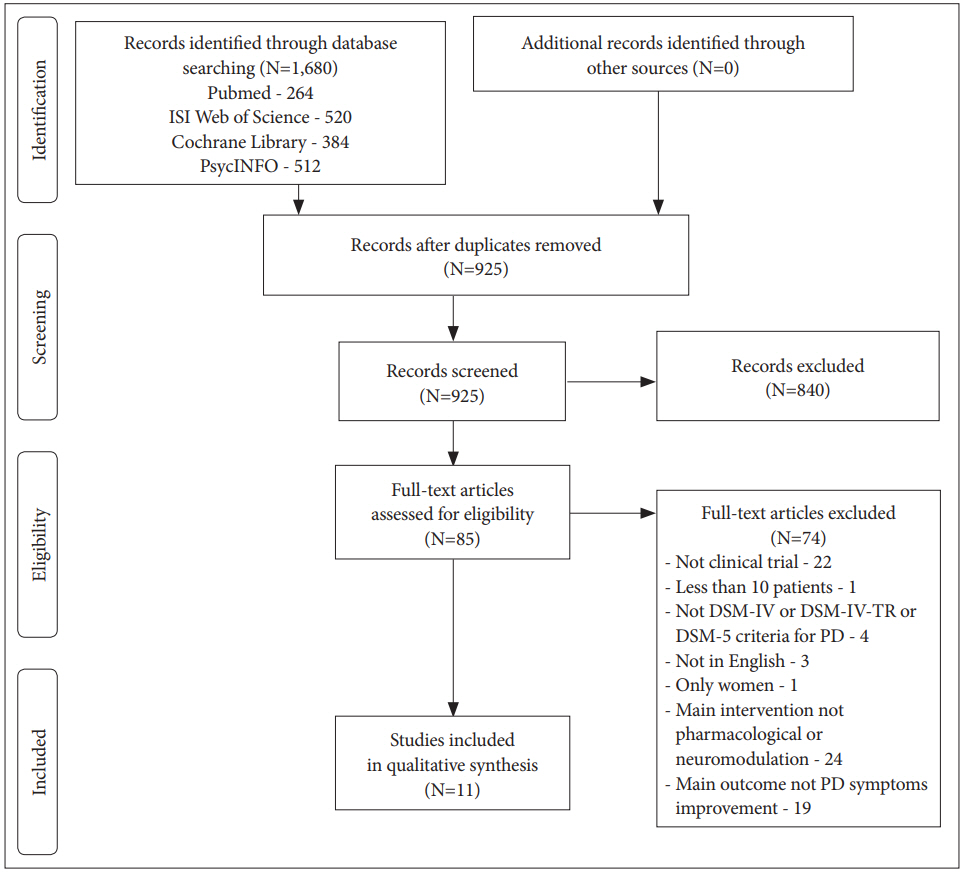
PRISMA diagram of study identification and selection process. DSM- IV: Diagnostic and Statistical Manual of Mental Disorders IV, DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders IV Text Revision, DSM-5: Diagnostic and Statistical Manual of Mental Disorders 5, PD: Panic Disorder.
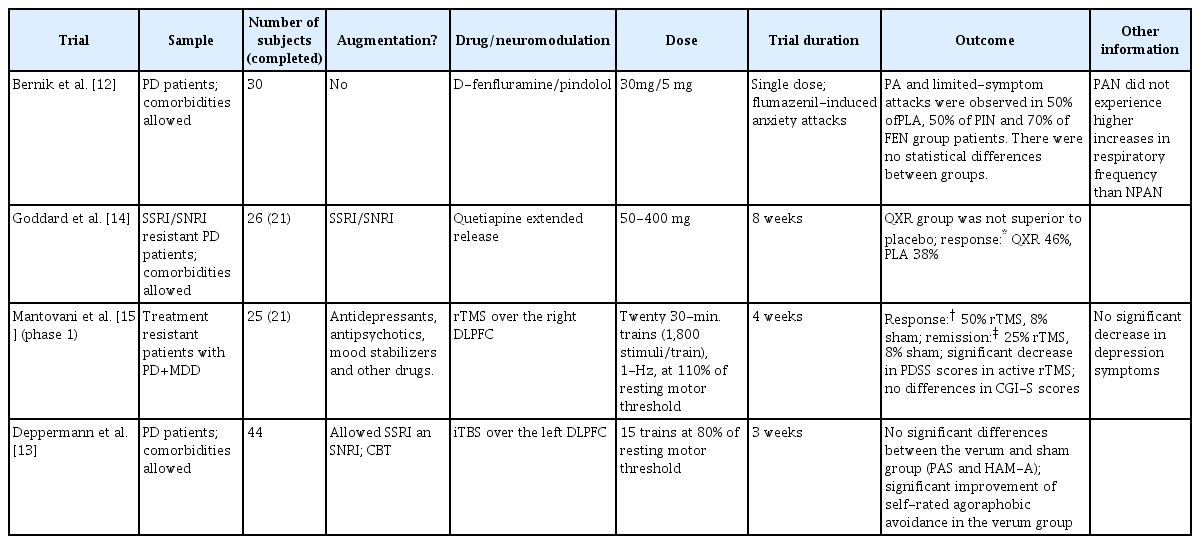
In the present review, 4 articles included RCTs [12-15], 3 articles included open trials [15-17] and 5 were comparative trials [18-22]. The article from Mantovani et al. [15] comprised two different clinical trials, phases 1 (RCT) and 2 (open trial), which were treated as independent trials. Two studies were on neuromodulatory treatments using rTMS. Four articles used DSMIV-TR criteria for PD; all the other articles used DSM-IV criteria for this disorder. The number of subjects in each study ranged from 21 to 190. The studied drugs were paroxetine, escitalopram, vortioxetine, tranylcypromine, quetiapine, dfenfluramine, pindolol, clonazepam and alprazolam. Five were augmentation trials. Trial duration ranged from 1 day (single-dose study) to 36 months. For heuristic purposes, we grouped the studies in four sections: RCTs, open clinical trials and comparative clinical trials.
Randomized controlled trials
Monotherapy studies
Bernik et al. [12] investigated whether acute enhancement of serotonin inhibits PAs. Single doses of either pindolol, d-fenfluramine or placebo were given to drug-free or never treated PD patients before a challenge test with intravenous infusion of flumazenil. All patients were symptomatic at the time with an average of 2.3 (SD: 0.9) PA per week. Anxiety and panic symptoms occurring before and during the challenge test were rated on the Emerging Panic Symptoms Scale (EPSS). Changes in scores of the EPSS following flumazenil challenge were not significantly different between the groups. Also, there were no differences between treatment groups in the heart rate or systolic/diastolic blood pressure. There were no differences in these measures between patients who had and those who did not have flumazenil-induced PAs.
Augmentation studies
Goddard et al. [23] conducted an augmentation trial with quetiapine extended release (XR) in a sample of SSRI-resistant, PD patients with comorbidities. For those who previously received an adequate (8 weeks or longer, in sufficient doses), ongoing SSRI therapy at intake, SSRI resistance was ascertained by a minimal improvement in CGI-I (≥3). Patients who were medication-free at intake were initially treated for 8 weeks with open-label, sertraline (50–200 mg/day); citalopram (20–40 mg/day) or escitalopram (10–20 mg/day). Following SSRI treatment, patients that had a <50% decrease from baseline in the Panic Disorder Severity Scale (PDSS) total score after the prospective SSRI trial, were classified as treatment-resistant. Only patients rated as moderately ill (CGI-S score ≥4) or worse were included. Anxiety and mood disorders (except bipolar disorder) were allowed. Patients on SSRI or SNRI were randomly assigned to receive either quetiapine XR or placebo. Baseline SSRI/SNRI doses were held constant throughout the 8-week trial. Quetiapine XR was not superior to placebo in this RCT.
Mantovani et al. [15] evaluated the efficacy of 1-Hz rTMS applied to the right dorsolateral prefrontal cortex (DLPFC) in patients with PD and comorbid major depressive disorder, who have not fully responded to conventional pharmacotherapy. The trial consisted of two phases. In phase 1, patients were randomly assigned to receive either active rTMS or sham stimulation 5 times a week, for 4 consecutive weeks. None of the patients reported significant side effects. The rTMS sessions were well tolerated. There were no seizures, neurological complications, or subjective complaints about memory or concentration impairments. Ratings of common side effects of rTMS showed no difference between the active and sham groups. Compared to the sham stimulation, active rTMS produced significant symptom improvements, with higher response and remission rates. There were no significant differences regarding the improvement in depression symptoms.
Intermittent Theta Burst Stimulation (iTBS), a new rTMS technique, was tested in the study from Deppermann et al. [13] iTBS or sham were applied over the left DLPFC in 15 daily sessions for 3 weeks, as an augmentation of a 9-week CBT protocol. No significant differences were found in the main outcome measures in the comparison between iTBS and sham stimulation, still the iTBS group showed a superior improvement on the agoraphobic avoidance scores compared to the sham condition. Findings from these studies are summarized in Table 1.
Open clinical trials
Augmentation studies
In the study from Mantovani et al. [15], at the end of phase 1 (4-week RCT), patients were offered the option of receiving open-label rTMS for additional 4 weeks (phase 2). Of 21 patients eligible to continue, 17 entered and completed the openlabel phase (9 initially randomized to active and 8 to sham). Three responders and one non-responder (to sham stimulation) refused to enter phase 2. The 9 patients initially randomized to receive active rTMS showed further improvements in phase 2 regarding panic and depressive symptoms, the response and remission rates were also increased. The 8 patients assigned to sham stimulation in phase 1 also showed significant betterment in phase 2, with improved response and remission rates.
Choi et al. [16] examined the efficacy of 24-week escitalopram treatment in terms of panic symptoms, functional disability, and quality of life in PD patients. The primary outcome measures were the remission and response rates based on the PDSS scores. Secondary outcome measures included changes from baseline in the Sheehan Disability Scale (SDS). A long-term efficacy of escitalopram in PD patients was demonstrated, with 87 of the 119 subjects attaining remission by the end of the study. A significant improvement in the total PDSS score was observed beginning from week 4, and the improvement continued progressively until the end of the study. Additionally, continuous significant improvements in all SDS sub-domains (work, social relationships, and responsibilities at home and with family) at baseline and at weeks 4, 12, and 24 were found.
The open trial with vortioxetine [17], despite its limitations, such as a small sample size and predominance of female subjects, indicated that this drug may be effective for treatmentresistant PD patients. The PDSS scores and the frequency of PA were significantly decreased by endpoint. There was also a trend towards an increased quality of life upon the completion of the study. Findings from these studies are summarized in Table 2.
Comparative clinical trials
Monotherapy studies
In the study from Buoli et al. [18], PD patients with or without agoraphobia were randomized to receive either paroxetine slow up-titration (increments of 2.5 mg/ day every 2 days) or standard up-titration (increments of 10 mg/day every week). The maximum dose for both groups was 20 mg/day. Clinical assessments with the Panic Attack Anticipatory Anxiety Scale (PAAS) and the Dosage Record and Treatment Emergent Symptom Scale (DOTES) were performed at baseline, 9 days and 18 days. No differences were found at end-point in the two treatment groups in terms of effectiveness and tolerability.
In the study from Nardi et al. [21] the objective was to compare the efficacy and safety of clonazepam and paroxetine in a 8-week trial with patients with PD. Efficacy parameters, which included number of PA, Clinical Global Impression Improvement (CGI-I) and Clinical Global Impression Severity (CGIS), were recorded at baseline, weeks 1, 2, 4, and 8. An one-week wash-out period was included to remove prior anti-panic medication. After initiation of drug treatment there was a dramatic decrease in the number of weekly PA in both treatment groups, which persisted over the course of the treatment period. Clonazepam had a faster onset of action than paroxetine, and at week 4, patients in the clonazepam group had significantly fewer PAs than those in the paroxetine group, but there were no significant differences between the two groups regarding CGI-S or CGI-I scores. By the endpoint the number of PA and CGI scores were much improved but there were no significant differences between the two groups. The rate of adverse events was higher in the paroxetine group compared to the clonazepam group. The most common adverse events in the clonazepam group were drowsiness/fatigue (57%), memory/concentration difficulties (24%), and sexual dysfunction (11%), while in the paroxetine group the most common were drowsiness/fatigue (81%), sexual dysfunction (70%), and nausea/vomiting (61%).
An extension of the 8-week study (above) was performed by Nardi et al. [22] for another 34 months, rendering a total of 36 months of treatment. Patients who responded to monotherapy in the first study continued with the same drug and dose. Response was defined as one PA or less per month, CGI-S scores of 1 or 2, and CGI-I scores of 1 or 2 at completion of the 8-week study. Nonresponders were invited to receive combination therapy with clonazepam and paroxetine for the longterm study. At baseline, the nonresponders group had more PA, higher CGI-S and CGI-I compared to the other two groups, but it had significant improvements with the combined treatment. At the 12-month, 24-month, and 36-month assessments, there were no significant differences between the three groups. During this trial, significantly more patients in the paroxetine group than those in the clonazepam group experienced sexual dysfunction, drowsiness/fatigue, diarrhea/constipation, dry mouth, excessive sweating, shaking/trembling/tremor, and nausea/vomiting, memory/concentration problems, insomnia/nightmares, headache, and paresthesia.
Nardi et al. [20] compared the efficacy and tolerability of 30 mg/day and 60 mg/day of tranylcypromine in PD patients with comorbid social anxiety disorder. The main instrument for clinical assessment was the Sheehan Panic and Anticipatory Anxiety Scale, from which the main efficacy measure -the percentage of patients with no PAs -was obtained. At the end of the study, more than 68% of the patients in each group were free of PA. The effect of the treatment with tranylcypromine was significant, but there were no significant differences between the 30 mg/day and the 60 mg/day groups. The adverse events were usually mild with a few of moderate intensity. No severe adverse event occurred during the study.
Marquez et al. [19] compared efficacy parameters between sublingual (ALP-SL) and conventional (ALP-CT) tablets of alprazolam in the treatment of acute phase of PD with and without agoraphobia. Patients who had score of 20 or higher on the HAM-A and who had been without pharmacological treatment in the 30 days prior the study were enrolled. Patients were assessed with HAM-A, PDSS, CGI, duration and intensity of PA. Both groups had significant improvements in all the measures but there were no significant differences between the two groups.
The final dose of alprazolam was less than 1.5 mg/day for both alprazolam formulations. Somnolence and sedation were the most common adverse events, without differences between the pharmaceutical forms. Findings from these studies are summarized in Table 3.
DISCUSSION
Evidence from research done in the last 8 years has shown efficacy and confirmed the antipanic properties of new and old medications. Pharmacological and neuromodulatory trials with the focus on improving PD symptoms were scant. RCT demonstrated efficacy of rTMS in only one of the two trials with this technique. Neither pindolol nor d-fenfluramine were effective in blocking flumazenil-induced panic attacks. Augmentation with quetiapine was not superior to placebo either. Open trials indicated that escitalopram, vortioxetine and TMS may be effective in the treatment of PD. Comparative trials did not demonstrate superiority from one drug to the other, but confirmed that tranylcypromine, paroxetine, clonazepam and alprazolam are effective drugs.
Low-frequency rTMS delivered to right DLPFC resulted in a significant clinical response [15]. Also, receiving a total of 8 weeks (phase 1 plus phase 2) (40 trains) of rTMS proved to be more effective than the 4-week treatment (20 trains) [15]. In the other rTMS trial [13], with 15 trains (3 weeks) of iTBS over de left DLPFC, the active condition was not superior to the sham condition. In a study published in 2007 [24], treatment-resistant PD patients received 10 trains of low-frequency rTMS in the right DLPFC, but active stimulation was not superior to sham stimulation. These findings provide preliminary indications that long treatments (i.e., 40 trains in 8 weeks) with low-frequency rTMS in the right DLPFC may be effective for PD, but not the short treatments. Neuromodulation studies in PD are scant and with serious limitations, for this reason the effectiveness of neuromodulation techniques is still unclear.
Several antidepressants demonstrated efficacy in the treatment of PD, including many SSRI, SNRI, tricyclic antidepressants and MAOI [7,25,26]. Vortioxetine was first reported in a study published in 2011 [27] and has been approved as a novel antidepressant for the treatment of MDD [28,29]. The results from the open-label study from Shah et al. [17] indicate that vortioxetine may be an effective drug for the treatment of PD, including treatment-resistant patients. The study from Nardi et al. [20] was the only tranylcypromine clinical trial with PD. This drug was effective and associated with few adverse events in PD patients. The current review confirmed de efficacy of paroxetine [21] and escitalopram [16].
The efficacy of anxiolytics in the treatment of anxiety is also well established [25]. Clonazepam, alprazolam, diazepam and lorazepam have been demonstrated to be effective in the treatment of PD [4,26,30,31]. In the present review alprazolam also showed to be effective in PD, with no differences between sublingual and conventional forms [19]. Nardi et al. [21] compared clonazepam and paroxetine in an 8-week and 36-months trial [22]. Although clonazepam group showed a faster onset of action than paroxetine at week 4, at the end of study there were no differences in CGI-S or CGI-I scores and no difference in number of PA. Overall, anxiolytics seem to be as effective as antidepressants, possibly with a faster onset of effect. Side effects of antidepressants are probably as severe as the side effects produced by anxiolytics.
Quetiapine showed anxiolytic properties in several studies [32,33], for this reason quetiapine was also a promising agent for the treatment of PD and treatment-resistant PD [5]. Quetiapine XR as used as augmentation for SSRI/SNRI in a RCT with a sample of SSRI-resistant PD patients, and was not found to be superior to placebo [23]. No other clinical trials with atypical antipsychotics in the treatment of PD were found in the timeframe of the current review. Studies published previously indicated that risperidone [34] is effective in the treatment of PD. Olanzapine [35] and aripiprazole [36] are also useful as augmentation strategies. Considering that evidences of effectiveness of atypical antipsychotics in the treatment of PD are few and these compounds probably induce more side effects than antidepressants and anxiolytics, atypical antipsychotics are not first-choice medications in the treatment of PD [25].
There are still few studies of head-to-head comparisons of drugs from different classes. There is also a scarcity of studies regarding neurostimulation in PD. A significant part of the studies published in the last 8 years has many serious limitations. The use of drugs other than the studied drug is a relevant confounding factor in many of these studies. Many studies included, in addition to pharmacological or neuromodulatory treatment, cognitive behavioral treatment, but for the most part, the main objective of these studies was the cognitive behavioral treatment and therefore, were not included in our study.
Strict inclusion methods were employed in this review, aiming to select only high quality studies published recently; therefore the number of included articles was reduced. Articles published before 2010 and studies with less than 10 PD patients were not contemplated in the current review, but they could include interesting new findings on this subject.
The current study confirmed the efficacy of tranylcypromine, paroxetine, clonazepam, alprazolam and escitalopram, and demonstrated efficacy for the new antidepressant vortioxetine. TMS, with duration of 4 or more weeks, may be effective in PD. Future studies should focus on neuromodulation techniques and new psychopharmacological compounds, comparing these new treatments to the well stablished effective treatments for PD.