1. World Health Organization. Depressive disorder (depression). Geneva: World Health Organization; 2023.
2. Korea Disease Control and Prevention Agency. Korea health statistics 2020: Korea national health and nutrition examination survey (KNHANES VIII-2). Cheongju: Korea Disease Control and Prevention Agency; 2022.
3. Fried EI, Epskamp S, Nesse RM, Tuerlinckx F, Borsboom D. What are ‘good’ depression symptoms? Comparing the centrality of DSM and non-DSM symptoms of depression in a network analysis. J Affect Disord 2016;189:314-320.


8. Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci 2008;31:464-468.


12. Bremmer MA, Deeg DJ, Beekman AT, Penninx BW, Lips P, Hoogendijk WJ. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry 2007;62:479-486.


13. Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 2011;73:114-126.


14. Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 2009;72:67-73.


15. Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev 2010;35:97-103.


16. Clow A, Hucklebridge F, Thorn L. The cortisol awakening response in context. Int Rev Neurobiol 2010;93:153-175.


17. Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 2009;80:265-278.


18. Veen G, van Vliet IM, DeRijk RH, Giltay EJ, van Pelt J, Zitman FG. Basal cortisol levels in relation to dimensions and DSM-IV categories of depression and anxiety. Psychiatry Res 2011;185:121-128.


19. Vreeburg SA, Hoogendijk WJ, DeRijk RH, van Dyck R, Smit JH, Zitman FG, et al. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology 2013;38:1494-1502.


20. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77-100.

21. Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573-583.


22. Giano Z, Ernst CW, Snider K, Davis A, O’Neil AM, Hubach RD. ACE domains and depression: investigating which specific domains are associated with depression in adulthood. Child Abuse Negl 2021;122:105335


23. Marganska A, Gallagher M, Miranda R. Adult attachment, emotion dysregulation, and symptoms of depression and generalized anxiety disorder. Am J Orthopsychiatry 2013;83:131-141.


25. Waugh CE, Koster EH. A resilience framework for promoting stable remission from depression. Clin Psychol Rev 2015;41:49-60.


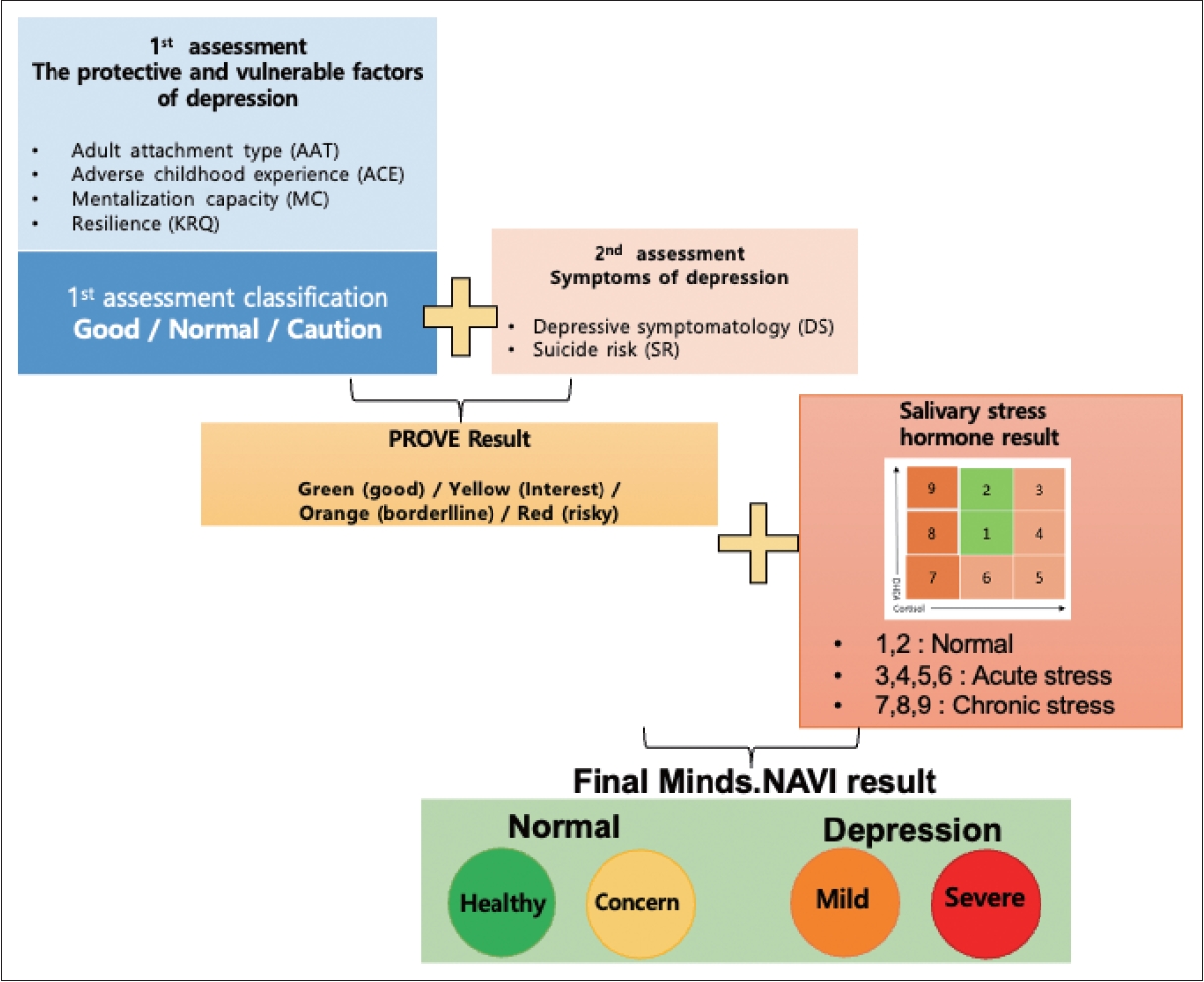
26. Lee JY, Choi SW, Jang SA, Ryu JS, Shin HK, Sim JY, et al. Development of the battery test for screening of depression and mental health: PROtective and Vulnerable factors battEry Test (PROVE). J Korean Neuropsychiatr Assoc 2021;60:143-157.


27. Reivich K, Shatté A. The resilience factor: 7 essential skills for overcoming life’s inevitable obstacles. Portland: Broadway Books; 2002.
28. Kim J. Resilience. Seoul: Wisdomhouse; 2019.
29. Shin WY, Kim MG, Kim JH. Developing measures of resilience for Korean adolescents and testing cross, convergent, and discriminant validity. Stud Korean Youth 2009;20:105-131.
30. Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Br J Psychiatry 2001;179:243-249.


31. Hong JP, Park SJ, Park S, Lim A, Jeon D. Reliability and validity study of the Korean self rating version of quick inventory of depressive symptomatology (K-QIDS-SR). Mood Emot 2013;11:44-50.
32. Yi JS, Bae SO, Ahn YM, Park DB, Noh KS, Shin HK, et al. Validity and reliability of the Korean version of the Hamilton depression rating scale (K-HDRS). J Korean Neuropsychiatr Assoc 2005;44:456-465.
34. Lee J, Shin C, Ko YH, Lim J, Joe SH, Kim S, et al. The reliability and validity studies of the Korean version of the perceived stress scale. Korean J Psychom Med 2012;20:127-134.
37. Hardeveld F, Spijker J, Vreeburg SA, Graaf RD, Hendriks SM, Licht CM, et al. Increased cortisol awakening response was associated with time to recurrence of major depressive disorder. Psychoneuroendocrinology 2014;50:62-71.


39. Stewart JW, Quitkin FM, McGrath PJ, Klein DF. Defining the boundaries of atypical depression: evidence from the HPA axis supports course of illness distinctions. J Affect Disord 2005;86:161-167.


40. Parker G, Roy K, Mitchell P, Wilhelm K, Malhi G, Hadzi-Pavlovic D. Atypical depression: a reappraisal. Am J Psychiatry 2002;159:1470-1479.


41. O’Keane V, Frodl T, Dinan TG. A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology 2012;37:1589-1599.


42. Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology 2005;30:568-576.


45. Fogelman N, Canli T. Early life stress and cortisol: a meta-analysis. Horm Behav 2018;98:63-76.


47. Gozansky WS, Lynn JS, Laudenslager ML, Kohrt WM. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic-pituitary-adrenal axis activity. Clin Endocrinol (Oxf) 2005;63:336-341.