Association Between White Matter Tract Integrity and Frontal-Executive Function in Non-Geriatric Adult Patients With Major Depressive Disorder
Article information
Abstract
Objective
This study investigated the association between white matter tract integrity and frontal executive function in adult non-geriatric patients with major depressive disorder (MDD) and healthy controls (HCs) using diffusion tensor imaging (DTI).
Methods
In total, 57 patients with MDD and 115 HCs participated in this study. We calculated the integrity of the white matter tracts using the Tracts Constrained by Underlying Anatomy tool (TRACULA) from FreeSurfer. We performed cognitive function tests. One-way analysis of covariance was used to investigate the DTI parameters as dependent variables; diagnosis of MDD as an independent variable; and age, sex, and education level as covariates. For correlation analysis between the DTI parameters and cognitive function tests, Pearson’s partial correlation analyses were performed in the MDD and HC groups.
Results
The patients with MDD showed significantly decreased axial diffusivity (AD) in forceps major (FMajor), left corticospinal tract (CST), left superior longitudinal fasciculus-parietal bundle (SLFP), right anterior thalamic radiation (ATR), right CST, right inferior longitudinal fasciculus (ILF) and right superior longitudinal fasciculus-temporal bundle (SLFT) and mean diffusivity (MD) in the left CST, right CST, and right SLFT compared to HCs. We found that non-geriatric patients with MDD showed a significant negative correlation between the response time in the Stroop task and the AD value of the FMajor.
Conclusion
Our findings suggest that impaired structural connectivity in the FMajor may be associated with cognitive dysfunction in non-geriatric patients with MDD.
INTRODUCTION
Major depressive disorder (MDD) is a mutual and burdensome medical illness that can cause diverse emotional and physical problems affecting people’s function at work and home [1]. During the coronavirus disease-2019 pandemic worldwide, almost 20% of the population has reported experiencing depression [2]. Symptoms of MDD include depressed mood, loss of enthusiasm, changes in appetite, fatigue, difficulty in thinking, and even thoughts of death. Among the diverse symptoms of MDD, cognitive dysfunction is a major psychopathology causing functional obsolescence in daily life [3]. The prevalence of cognitive dysfunction in MDD is estimated to range from 33% to 75% [4], with up to 50% of patients with MDD experiencing residual cognitive manifestations even after achieving remission from their depressive episodes [5]. Cognitive dysfunction affects attention, executive function, learning, and working memory. This deficit is the primary symptom of functional impairment in patients with MDD [6]. Cognitive decline in learning, memory, executive function, and attention are frequently observed in patients with major depressive episodes. Combined with depressed mood and anhedonia, cognitive dysfunction is a major obstacle for patients in maintaining their functional abilities [7].
Previous studies have investigated the neural correlations between cognitive dysfunction and MDD. Ge et al. [8] found lessened connection of the right intermediate hippocampus with limbic regions between patients with treatment-resistant depression and healthy controls (HCs) using resting-state functional magnetic resonance imaging (fMRI). Another study found that geriatric patients with depression showed cognitive dysfunction, as measured by the N-back test of working memory, with decreased later frontal and parietal activation in fMRI compared to non-depressed geriatric HCs [9]. For structural MRI studies, Zacková et al. [10] performed a meta-analysis of 48 voxel-based morphometric (VBM) studies in patients with MDD, mild cognitive decline, and age-matched controls. This study found that patients with MDD and mild cognitive impairment (MCI) shared volume decreases in the insula and superior temporal gyrus (STG), which might reflect communication deficiencies and social withdrawal, which are known risk factors for both MCI and MDD. Although microstructural deviations in white matter (WM) play a vital role in the pathology of MDD [11,12], few studies have observed the correlation between cognitive dysfunction and WM integrity in MDD. A diffusion tensor imaging (DTI) study [13] assessed the associations between WM integrity and cognitive functioning in euthymic patients with MDD and bipolar disorder (BD) compared with HCs. The results showed that lower fractional anisotropy (FA) of the corpus callosum body was associated with lower continuous attention and set-shifting scores compared to the other groups. Meinert et al. [14] conducted a DTI study on the association between brain structure and cognitive function in patients with MDD, and found an association between reduced cognitive function and lower FA values in a bulky bilateral cluster involving widespread frontotemporal association fibers. Another DTI study using tract-based spatial statistics (TBSS) found that Stroop interference interrelated positively with FA in the left caudal anterior cingulate cortex (cACC) in HCs, but not in patients with MDD using TBSS [15].
Few studies have been performed on non-geriatric populations aged 19–64 years, which is the most active working population. Furthermore, considering cognitive dysfunction is one of the depression symptoms that has the greatest impact on psychosocial functioning [16] and also remains long after remission [17], disability in this population can cause socioeconomic burden. Cognitive dysfunction also has a significant impact on quality of life, which is one if the main discomfort patients with MDD experience [18].
TBSS detects spatially consistent effects, so that voxels are detected as significant unit to compare reduced FA between groups. Therefore differences in voxel unit can be shown meaningful even if they themselves have very small effect sizes [1].9 However, probabilistic tractography, such as Tracts Constrained by UnderLying Anatomy tool (TRACULA) which we used in this research, is more sensitive to tract-specific FA differences that may be obscured by normalization to standard space, that it allows for more readily interpretable effect sizes, and that it can generate specific tract and function-specific hypotheses [20]. Using TRACULA, this study aimed to investigate the association between WM tract integrity and frontal executive function in non-geriatric adult patients with MDD aged 19–64 years and in HCs. We hypothesized that there would be an inverse correlation between the cognitive function test and WM tracts involved in frontal-executive function, such as the corpus callosum, cingulum, uncinate fasciculus (UF), and anterior corona radiata [21].
METHODS
Participants
A total of 57 patients with MDD and 115 HCs were included in this study. Participants were enrolled from the outpatient psychiatric clinic of Korea University Anam Hospital in Seoul, Republic of Korea, between June 2019 and February 2022. The participants included in the study were adults aged 19–64 years. The patients were diagnosed with MDD by two board-certified psychiatrists (K.M. Han & B.J. Ham) using a Structured Clinical Interview according to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders, Clinician Version (SCID-5-CV) [22]. We excluded patients with 1) comorbidity of any other major psychiatric disorder, 2) psychotic features such as delusions and hallucinations, 3) high suicidality requiring immediate inpatient treatment, 4) a history of major medical conditions, 5) primary neurological illness, or 6) any contraindication for neuroimaging. The duration of illness was assessed as the lifetime cumulative number of months of depressive episode(s) using the life-chart methodology. A total of 115 HCs aged 19–64 years were recruited from the community through public advertisements. Two board-certified psychiatrists assessed HCs and confirmed the absence of current or previous psychiatric disorders. The same exclusion criteria were applied in the HC group. The extent of mood symptoms in all patients was assessed using the 17-item Hamilton Depression Rating Scale (HDRS) [23]. All participants were right-handed according to the Edinburgh handedness test. The demographic and clinical characteristics of the patients included in the neuroimaging analyses are presented in Table 1. At the time of study engagement, 12 patients were drug-naïve and 45 patients were taking psychotropic medication. The study protocol was approved by the Institutional Review Board of Korea University Anam Hospital (IRB No. 2019AN0174). All participants provided written informed consent before participation, in accordance with the Declaration of Helsinki.
Image acquisition
MRI scans were collected at the Korea University Magnetic Resonance Image Center using a 3.0-Tesla Trio whole-body imaging system (Siemens Healthcare GmbH, Erlangen, Germany). DTIs were obtained using an echo-planar imaging sequence with the following parameters: repetition time=6,300 ms; echo time=84 ms; field of view=230 mm; 128×128 matrix; 3-mm slice thickness with no gap; transversal orientation; voxel size: 1.8 mm×1.8 mm×3.0 mm; diffusion directions=20; number of B0 images=1; number of slices=50; b-values: 0 and 600 s/mm2; acceleration factor (iPAT-GRAPPA)=2 with 38 reference lines for phase encoding direction and 6/8-phase partial Fourier. Artifacts in the images derived from the MRI system or head motion were visually checked after the MRI scanning. If artifacts were observed, the subject’s MRI scans were repeated.
Image preprocessing and data extraction
To calculate the integrity of the WM tracts, we used a design for the automated probabilistic reconstruction of major WM tracts, TRACULA (from FreeSurfer [23]), to reassemble 18 major WM paths at each participant’s time point. TRACULA analysis was performed as previously described [24]. We enrolled DTIs in the b=0 images for simple head motion and eddy currents, and underwent enrollment transformation using FreeSurfer’s bbregister [25,26]. Using this information, the mapping of cortical parcellation and subcortical segmentation in the DTIs of each participant was reassembled using FreeSurfer, and the FSL’s Bayesian estimation of diffusion parameters was achieved using sample techniques [24]. A ball-and-stick diffusion model was used to determine the local diffusion orientation for each participant. We used TRACULA to estimate the probability distribution of the 18 major WM tracts of each participant’s ball-and-stick model and to label the cortical and subcortical segments. The 18 WM tracts were as follows (Figure 1): forceps major (FMajor) and forceps minor (FMinor), anterior thalamic radiation (ATR), cingulum-angular bundle (CAB), cingulum-cingulate gyrus bundle (CCG), corticospinal tract (CST), inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus-parietal bundle (SLFP), superior longitudinal fasciculus-temporal bundle (SLFT), and the UF in both hemispheres. Finally, four DTI parameters, FA, mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD), were obtained from the delineated individual WM tracts of the participants using FSL’s dtifit function (http://www.fmrib.ox.ac.uk/fsl).
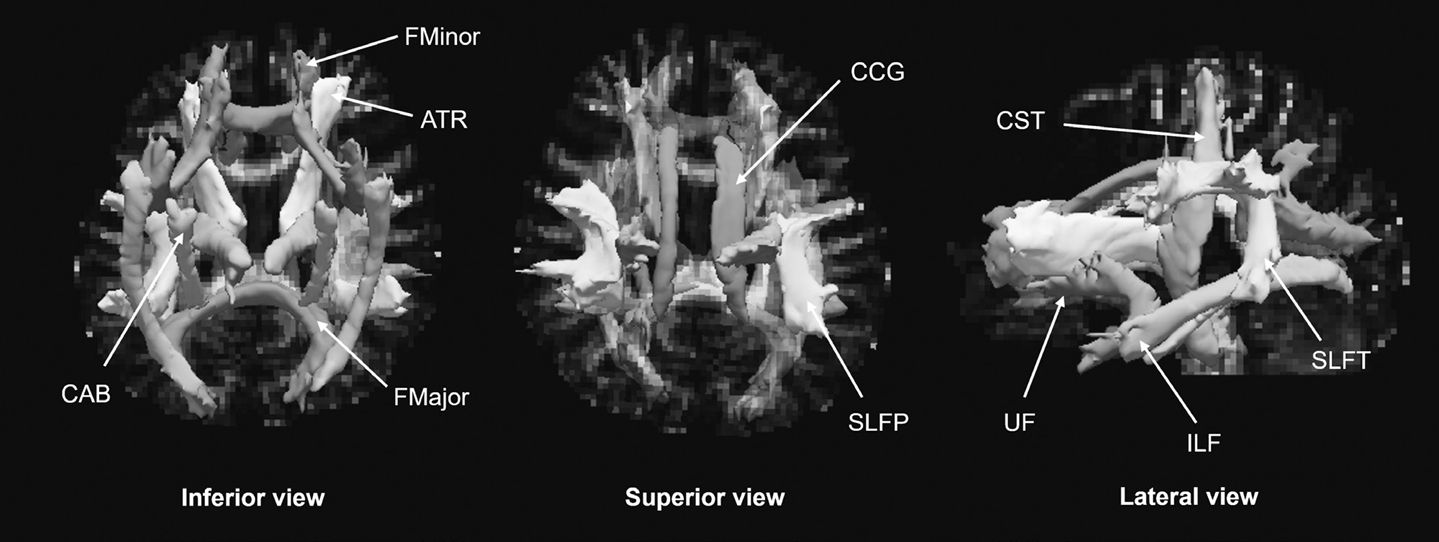
Three-dimensional reconstruction of 18 major white matter (WM) tracts in the Tracts Constrained by UnderLying Anatomy tool (TRACULA). FMajor, forceps major; FMinor, forceps minor; ATR, anterior thalamic radiation; CAB, cingulum-angular bundle; CCG, cingulum-cingulate gyrus bundle; CST, corticospinal tract; ILF, inferior longitudinal fasciculus; SLFP, superior longitudinal fasciculus-parietal bundle; SLFT, superior longitudinal fasciculus-temporal bundle; UF, uncinate fasciculus.
The FA index, which is a summary measure of microstructural integrity, is the most widely used DTI parameter. FA is highly sensitive to microstructural changes in axonal fibers. Higher values of the FA index represent either an expansion of axon fiber number and size or a reduction in the density of crossing fibers [27]. The MD index is known from previous studies represents damage, such as cellularity, necrosis, and edema; the RD index represents myelination; and the AD index represents axonal pathologies, which may reflect the WM tissue microstructure [28]. The MD, RD, and AD indices are thought to supplement the FA index in explaining possible underlying alterations in WM tissue microstructure [29,30].
Assessments of cognitive functions
Executive functions play an essential role in the planning and initiation of independent activities, self-monitoring of performance, inhibition of in appropriate responses, switching between tasks, and planning and control of complex motor and problem-solving responses [31]. Participants were tested on the digit span, matrix reasoning, trail-making, and Stroop tasks to measure attention, processing speed, response inhibition, working memory, and cognitive flexibility of cognitive functions. The digit span test and matrix reasoning test were extracted from the Wechsler Adult Intelligence Scale IV (WAIS-IV) [32]. These two tests were selected because they are ideal and pragmatic cognitive function tests, considering the limited time, and to increase the reliability and validity of the study [32].
The digit span test is a systematized memory task consisting of two parts, digit forward and digit backward, which measures short-term memory [33], information processing, and working memory [34]. This test is composed of two parts, including digit forward and digit backward. Digit forward is auditoryverbal measure of simple span of attention which is associated with language processes in the left hemisphere [35], and digit backward is cognitive manipulation of increasingly longer strings of digits which is associated with visual-spatial skills in the right hemisphere [36] and frontal lobe functioning [37]. The digit span test was used to assess working memory. The participants were instructed to repeat the task after listening to a set of numbers and pronouncing the numbers backward and in ascending order. If the participant was correct, the score was 1; otherwise, it was 0. The digit span test consisted of three parts, each with 16 trials, with an overall score of 48 (one point for every correct answer). If any of the subset scores was zero, the test was excluded.
Matrix reasoning was used to measure performance IQ and test perceptual organizational ability. Three sets of drawings were shown in the matrix reasoning tests and participants were asked to anticipate which drawing was to follow. If a participant provided incorrect answers three times consecutively, the test was excluded. Matrix reasoning test measure non-verbal reasoning skills, abstract thinking, problem-solving abilities, and perceptual organization of frontal-executive function [38]. During the test, the participants were presented with a pattern or matrix of shapes with one missing element. Then, they were required to identify the missing pieces that fit logically and complete the pattern according to the underlying rules or relationships within the matrix. Strong performance on the matrix reasoning test is known to suggest good abstract reasoning and problem-solving abilities, which are important components of cognitive functioning and intelligence [38].
A trail making test (TMT) requires rapid and efficient integration of attention, visual scanning, and cognitive sequencing. TMT was conducted to assess visual attention, processing speed, and cognitive flexibility [39]. The test consisted of two parts: the TMT-A and TMT-B. The TMT-A indicates visual search and motor speed, whereas the TMT-B reflects visual search and cognitive alternations [40]. In the first part (i.e., TMT-A), the participant was instructed to connect a set of numbers from 1 to 25 randomly written on paper in one step. In the second part (i.e., TMT-B), the participant is asked to link a set of Korean letters and numbers in an ascending order, alternatively (e.g., 1-가-2-나-3-다…) until 25. The TMT-A and TMT-B scores were documented by performance time, with a longer time suggesting poorer cognitive function.
The Stroop task was performed to measure the ability to repress cognitive interference that occurs when the operation of a certain stimulus feature delays the concurrent operation of a second stimulus attribute, known as the Stroop effect [41]. The Stroop task measures cognitive functions such as attention, processing speed, cognitive flexibility [42] and working memory [43]. The Stroop task was composed of three parts, where in each part, the participant names the colors of an assigned circle (red, blue, green), words that are not related to colors (e.g., saying ‘blue’ from ‘spring’ in blue color) and lastly words that are written in another colors, different from the word itself (e.g., saying ‘yellow’ from a yellow colored ‘green’). The scores for the TMT and Stroop tasks were measured by reaction time and counting the number of wrong answers, which suggested that higher scores were associated with poor cognitive function.
Statistical analyses
We compared the demographic and clinical characteristics of patients with MDD and HCs using the independent t-test (for continuous variables) and chi-square test (for categorical variables). An independent t-test was used to compare cognitive function between the two groups.
For neuroimaging analysis, the comparison of DTI parameters (i.e., FA, MD, RD, and AD) between the MDD and HC groups was performed using a one-way analysis of covariance with age, sex, and years of education as covariates. Correlation analyses were performed between DTI parameters and cognitive function tests in the MDD and HC groups using Pearson’s partial correlation analysis. In the MDD group, Pearson’s partial correlation analysis was conducted with age, sex, years of education, HDRS score, duration of illness, and medication (drug-naïve patients were coded 0; patients taking psychotropic medication were coded 1) as covariates. For the HC group, a Pearson’s partial correlation analysis was conducted with age, sex, and years of education as covariates. In all neuroimaging analyses, Bonferroni correction was applied for multiple comparison correction: p<0.05/72 (18 WM tracts× 4 parameters)=0.000694. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 25.0; IBM Corp., Armonk, NY, USA).
RESULTS
Sociodemographic and clinical characteristics of participants
The demographic and clinical characteristics of the 57 patients with MDD and the HCs are shown in Table 1. The MDD and HC groups did not differ significantly in terms of age or sex (all p>0.1). The HC group had significantly more years of education than the MDD group (p=0.001), and the HDRS score was significantly higher in the MDD group (p<0.001). The mean duration of illness in the MDD group was 34.28±20.70 months (Table 1).
Comparison of cognitive function tests
For the cognitive function tests, compared with HCs, the MDD group had significantly lower scores in the digit span test and matrix reasoning test and longer response times in TMT-A and TMT-B (p<0.05) (Table 1). For the Stroop task test, patients with MDD showed longer response time compared to HC group, however, the difference only showed a trend of statistical significance (p=0.065) (Table 1).
Comparison of DTI parameters between patients with MDD and HCs
The MDD group showed no substantial difference in FA values related to the HC group (Table 2). However, there were substantial differences in the AD, RD, and MD among the WM tracts (Table 2). For AD, the MDD group presented significantly reduced AD in the FMajor, left CST, left SLFP, right ATR, right CST, right ILF, and right SLFT, all of which survived after Bonferroni correction (p<0.000694). For MD and RD, patients with MDD showed significantly reduced MD in the left CST, right CST, and meaningfully lower RD in the left CST (Table 2).
Correlation of DTI parameters with Stroop task within MDD and HC groups
In the correlation analyses between DTI parameters and the scores of the Stroop task in the MDD group, there was a significant negative relationship between the response time of the Stroop task and the AD value of FMajor (r=-0.553, p=2.58×10-5) (Table 3), which remained meaningful after Bonferroni correction (i.e., p<0.000694). The FMajor group showed significantly reduced AD in the MDD group compared with the HC group, as shown in Table 2. In addition, the FA of FMajor showed a negative correlation with the response time of the Stroop task (r=-0.422, p=0.002), which was not significant after Bonferroni correction. No significant correlations were observed in the HC group (Table 3).
Correlation of DTI parameters with the other cognitive tests within MDD and HC groups
For cognitive tests other than the Stroop task, the AD of FMajor showed a negative relationship with the response time of the TMT-A (r=-0.416, p=0.002), however, it was no significant after Bonferroni correction. In the HC group, there were no significant correlation between cognitive tests and DTI parameters, which remained significant after Bonferroni correction (Table 3).
DISCUSSION
This study aimed to identify the association between cognitive function and WM tract integrity in adult non-geriatric patients with MDD and in HCs. We then performed cognitive function tests (digit span test, matrix reasoning, and TMT-A and -B) to evaluate each domain of frontal executive function (working memory, response inhibition, and cognitive flexibility), processing speed, and attention in every participant, as well as DTI-derived scalar values (FA, MD, RD, and AD) for the 18 WM tracts using the TRACULA method. We observed a significant negative correlation between response time in the Stroop task and the AD value of FMajor, which remained significant after Bonferroni correction. The AD values of the FMajor and TMT-A response times and the FA values of the FMajor and response of the Stroop task showed negative correlations. Although these findings were not significant after the Bonferroni correction, they were still considered important.
Additionally, there were significant differences in the AD, RD, and MD in several WM tracts between patients with MDD and HCs. The AD values of the FMajor, left CST, left SLFP, right ATR, right CST, right ILF, and right SLFT were significantly lower in patients with MDD than in HCs. The MD values of the left CST, right CST, and right SLFT were significantly reduced and the RD values of the left CST were lower in patients with MDD than in HCs.
There are many studies on the correlation between cognitive function and WM integrity in older patients with MDD (i.e., those aged >65 years) compared to HCs. A previous study of older patients with MDD showed significant associations between FA values and the Stroop task in multiple frontostriatal limbic regions, comprising the WM lateral to the anterior and posterior cingulate cortices and the WM in the prefrontal, insular, and parahippocampal regions [44]. These results indicate that microstructural changes in the frontostriatal-limbic networks are associated with frontal executive dysfunction in late-life depression. Another study demonstrated that FA in the bilateral SLF and right CST is positively related to better cognition as measured by the initiation/perseveration subscale of the Dementia Rating Scale in patients with late-life depression (aged 60–90 years) [45].
A meta-analysis was conducted in adolescents and young adults, comparing differences in WM integrity between patients with MDD (mean age 23.00 years) and HCs (mean age 22.37 years). Patients with MDD showed significantly lower FA values in the corpus callosum, extending to the left anterior thalamic and left corticospinal projections [46]. However, the correlation between cognitive function and WM integrity has not been analyzed.
We found that, in the MDD group, there was a significant negative correlation between the response time of the Stroop task and the AD value of FMajor. The Stroop test assesses the speed of visual investigation, working memory, and inhibition of cognitive interference among frontal executive functions [47]. In agreement with our findings, other neuroimaging studies have found a significant correlation between FMajor WM integrity and cognitive function in patients with MDD. A DTI study showed lower FA values in the global WM fiber structure in patients with MDD than in HCs, which was correlated with cognitive dysfunction [12]. Rizk et al. [15] found a positive correlation between WM integrity and Stroop interference in unmedicated young adult patients with MDD. They found lower FA values in the left genu of the corpus callosum, right splenium of the corpus callosum, and left anterior cingulate WM in the MDD group than in the HC group. There was also a significant relationship between reduced FA values of the anterior callosal fibers in the MDD and BD groups compared to those in the HC group and lower raw scores on the digit sequencing task and symbol coding in the MDD group [48].
FMajor is a WM tract that radiates from the corpus callosum, runs through the splenium, and connects to the posterior portions of the occipital lobes. Anatomically, the corpus callosum plays a key role in connecting the cerebral hemispheres and coordinating higher cognitive functions in the brain [49]. In a large multi-site study examining WM integrity in patients with MDD and HC, a subtle but widespread decrease in FA was observed in adults with MDD. Among the WM tracts, the largest differences were observed in the corpus callosum and corona radiata [50]. The FMajor, which connects the corpus callosum to the occipital lobes, may contribute to the visuospatial function of cognition in relation to topographical projections. Therefore, our results showing a significant negative correlation between the response time of the Stroop task and the AD value of FMajor in patients with MDD agree with the cognitive function of FMajor already demonstrated in previous research.
The AD parameter in the DTI study reflects the rate of diffusion of water molecules in neuronal tissues. It is influenced by the microstructural properties of the tissue, such as axon diameter, myelination, and tissue integrity [11]. A lower AD value indicates restricted diffusion, which can be associated with various conditions or pathologies affecting the WM, such as axonal injury, demyelination, or neurodegenerative diseases that result in the loss or disruption of axonal fibers. In this study, we found a significant negative correlation between the response time on the Stroop task and the AD value of FMajor. From this finding, we can conclude that there is microdamage such as demyelination, axonal injury, or neurodegeneration occurs in the major tracts of patients with MDD, thus affecting frontal executive function.
In this study, the AD of FMajor was reduced in the MDD group compared to the HC group, and there was an inverse correlation between the response time of the Stroop test and the AD of FMajor only within the MDD group. However, we did not find significant between-group differences in the FA. This result might be due to prominent decrease of the AD, which reflects axonal injury, in the MDD group, whereas this group showed significant decrease of the MD in several WM tracts rather than increase. Given that the MD (λ1+λ2+λ3/3) contains AD (λ1) and RD (λ2+λ3/2), decrease of in the MD in the MDD group may be due to the impact of prominent decrease of the AD in the MDD group. In summary, given that FA is a summary variable of AD, RD, and MD, the lack of significant findings in FA might be attributed to a prominent reduction in MDD-related AD, which was accompanied by a decrease in MD in patients with MDD. In addition, the relatively small sample used in the present study may have contributed no significant findings concerning FA.
This study has several limitations. First, although the FA of FMajor showed a negative correlation with the response time of the Stroop task (r=-0.422, p=0.002) (Table 3), it did not remain significant after Bonferroni correction. This might be due to the small sample size of patients with MDD (57 participants) compared to 117 HCs. In addition, we could not exclude the confounding effects of psychotropic medications. Of the patients with MDD, only 12 were drug-naïve, and 45 were drug-treated. The use of antidepressants and a combination of antipsychotics may have protective effects on the brain microstructure [51]. Finally, this study used a cross-sectional design, and we could not elucidate the causal relationship between reduced AD of the FMajor and frontal executive dysfunction in MDD.
To the best of our knowledge, this is the first study to report an association between WM integrity and frontal-executive function in non-geriatric adult patients with MDD and HCs. Although other studies have examined the correlation between decreased FA values and cognition, this is the first study to identify a negative correlation between AD values and frontal executive function. The results of the present study suggest that impaired structural connectivity resulting from actual pathologies, such as the microdamage indicated above in FMajor, may be associated with frontal-executive dysfunction in non-geriatric adult patients with MDD. It can be used as a neuroimaging marker to detect cognitive dysfunction in patients with MDD.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
Kyu-Man Han, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Byung-Joo Ham, Kyu-Man Han. Data curation: Youbin Kang, Aram Kim. Formal analysis: Kyu-Man Han, Woo-Suk Tae. Funding acquisition: Byung-Joo Ham. Investigation: Byung-Joo Ham, Kyu-Man Han. Methodology: Kyu-Man Han, Woo-Suk Tae. Project administration: Byung-Joo Ham, Kyu-Man Han. Resources: Byung-Joo Ham, Kyu-Man Han. Software: Woo-Suk Tae. Supervision: Byung-Joo Ham, Kyu-Man Han. Validation: Kyu-Man Han. Visualization: Kyu-Man Han. Writing—original draft: Joo-Yeon Ahn. Writing—review & editing: Byung-Joo Ham, Kyu-Man Han.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020M3 E5D9080792; NRF-2022R1A2C2093009; No. 2022R1A2C4001313) and by the Government-wide R&D Fund for Infections Disease Research (GFID), funded by the Ministry of the Interior and Safety, Republic of Korea (grant number: 20015024).



