Associations of TNF-RII rs1061622 With Post-Traumatic Stress Disorder and Their Interplays on Serum Lipids Levels in Adolescents
Article information
Abstract
Objective
To verify effects of rs1061622 at tumor necrosis factor-α receptor II (TNF-RII) gene (TNF-RII) on post-traumatic stress disorder (PTSD) and its interactive effects with PTSD on serum lipids levels in adolescents.
Methods
PTSD was measured by PTSD Checklist-Civilian Version (PCL-C) in 699 adolescent survivors at 6 months after Wenchuan earthquake in China. A polymerase chain reaction and restriction fragment length polymorphism assay were utilized for TNF-RII rs1061622 genotyping followed by verification using DNA sequencing. Serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol were tested using routine methods.
Results
G (deoxyguanine) allele carriers had higher PCL-C scores than TT (deoxythymidine) homozygotes in female subjects. Female adolescents had higher PCL-C scores than male subjects in TT homozygotes. Predictors of PTSD prevalence and severity were different between G allele carriers and TT homozygotes. Subjects with PTSD had lower TG, TG/HDL-C, TC/HDL-C, and higher HDL-C than adolescents without PTSD in male G allele carriers. G allele carriers had higher TG/HDL-C and TC/HDL-C than TT homozygotes in male adolescents without PTSD, and lower TG and TG/HDL-C in male PTSD patients. G allele carriers had higher TG than TT homozygotes only in female adolescents without PTSD.
Conclusion
These results suggest reciprocal actions of TNF-RII rs1061622 with other factors on PTSD severity, interplays of TNF-RII rs1061622 with PTSD on serum lipid levels, and novel treatment strategies for PTSD and comorbidities of PTSD with hyperlipidemia among adolescents with different genetic backgrounds of TNF-RII rs1061622 after experiencing traumatic events.
INTRODUCTION
Post-traumatic stress disorder (PTSD), a psychological response to traumatic situations, is a psychological disorder easily observed in common populations [1]. The most common symptoms of PTSD, including hyperarousal, unpleasant feelings, avoidance, weak focus, numbing, and trouble in unambiguously remembering features of traumatic experiences, appeared weeks or months after traumatic events such as earthquakes [2,3].
In fact, PTSD is not only a psychological disorder. It has been displayed that PTSD is closely related to unfavorable lipid profiles. Several lines of evidence demonstrated that patients with PTSD exhibited significantly higher levels of triglyceride (TG) [4], total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) [5] and lower levels of high-density lipoprotein cholesterol (HDL-C) [6,7]. Contrarily, a previous study also displayed decreased levels of LDL-C in PTSD patients when compared with healthy controls [8]. Nevertheless, the levels of TC, HDL-C, LDL-C [9], and TG [10] in PTSD patients were not observed to be significantly different from those without PTSD or healthy control subjects in other studies. Therefore, the relationship between PTSD and lipid profiles is complex and the contradictions in the previous studies have not been clarified yet.
The pathophysiological mechanism of PTSD has not been fully elucidated yet. However, the reduced volume of the hippocampus caused by inflammation was considered to be associated with PTSD symptoms [11]. While increased levels of tumor necrosis factor α (TNF-α) and its receptor tumor necrosis factor-α receptor II (TNF-RII) were revealed as hippocampal volume reducers [12,13], the elevation of TNF-α and TNF-RII was identified as peripheral indicators of PTSD [14,15]. Moreover, the severity of PTSD was detected to be related to increased levels of TNF-RII [16,17]. However, previous studies also displayed that TNF-RII may not play a role in the pathophysiology and development of PTSD symptoms in Gulf War veterans with PTSD or male German soldiers [11,16].
TNF-RII was also found to be closely related to dyslipidemia and cardiovascular disease. A cohort study of 607 diabetes patients showed higher circulating TNF-RII could predicate incident cardiovascular events adjusting for age, sex, glomerular filtration rate, and urinary albumin/creatinine ratio [18]. Meanwhile, there was a negative correlation between TNF-RII level and HDL-C in healthy subjects with a family history of coronary disease [19]. However, overweight subjects with type 2 diabetes mellitus after hypoglycemic therapy showed significantly decreased levels of fasting TC, LDL-C, and TNF-RII [20]. Obviously, more efforts are needed to clarify the relationship between TNF-RII and serum lipid profiles.
Polymorphisms have been revealed in the TNF-RII gene (TNF-RII). TNF-RII rs1061622 (T676G) is the one at exon 6. The replacement of deoxythymidine (T) with deoxyguanine (G) at 676 nucleotide results in an amino acid variation in the fourth extracellular cysteine-rich domain from methionine to arginine at 196 in the protein sequence [21]. TNF-RII rs1061622 has been demonstrated to be associated with psychiatric disorders. The G allele of TNF-RII rs1061622 was found to be associated with a substantially higher risk of paranoid schizophrenia [22]. Reduced recovery of depression in female T allele carriers of TNF-RII rs1061622 at an earlier stage after the earthquake [23]. Meanwhile, the relationship between the G allele and high symptom burden for pain, depressed mood, and fatigue symptom cluster was found in lung cancer patients [24]. However, the association of TNF-RII rs1061622 with PTSD, as well as their interplays on serum lipids has not been reported before.
Therefore, to explain inconsistent or even contradictory relationships among TNF-RII, PTSD, and serum lipid levels, and further explore the regulatory mechanism of serum lipid levels by PTSD, we hypothesized that interplays may occur between TNF-RII rs1061622 and PTSD, and further influence serum lipid levels. To test the hypothesis, we examined the relationship between serum lipid profiles, TNF-RII rs1061622 and PTSD in Chinese adolescents at 6 months after the 2008 Wenchuan earthquake. This population was selected because their prevalence increased steadily [25] and they were more easily affected by stresses such as earthquakes [26].
METHODS
Study population
A boarding high school, situated at a 10 km distance from the epicenter of the Wenchuan earthquake, was selected for the present study. Six months after the earthquake, students studying in grade 11 who lived and studied in temporary buildings due to the damage of the teaching halls and dormitories were recruited as participants. The students who were taking any medication were excluded. A total of 699 students were enrolled in the current study as they had accomplished all the measures and provided blood samples. They are Chinese Han. The study was approved by the Human Ethics Committee of Sichuan University (3011012). Informed consent was obtained from all participants and their guardians with a full explanation of the study.
Measurements
The approach included two parts in the current study [27]. Briefly, the first part included the evaluation of demographic characteristics (sex and age), traumatic features (injuries to self, damage to family property, extent of damage to family housing, death or injury of family members, and exposures directly to death or injury of family members or home destruction), personal history (previous trauma experiences), and family backgrounds (numbers of family members, only-child status, education levels of parents, and family history of psychiatric disorders) [28], while the second part was used to measure PTSD symptoms by PTSD Checklist-Civilian Version (PCL-C), which had been used commonly and showed high internal consistency in adolescents [29]. According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [30], an adolescent with a PCL-C score of 38 or greater from a total score of 85 in a 17-point self-reporting questionnaire was classified as a subject with PTSD [31]. Cronbach’s α coefficient of the PCL-C ranged from 0.891 to 0.894 in the present study. Serum TC, TG, HDL-C, and LDL-C levels were measured by routine methods, while TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C were calculated.
DNA extraction and genotyping
Genomic DNA was extracted from peripheral leucocytes using a commercial DNA extraction kit (Tiandz, Mianyang, China) and stored at -80°C. The genotypes of TNF-RII rs1061622 were determined by the polymerase chain reaction-restriction fragments length polymorphism method. TNF-RII rs1061622 containing DNA of 242 base pairs was amplified by the following primers [32]: forward 5'-ACT CTC CTA TCC TGC CTG CT-3'; and reverse 5'-TTC TGG AGT TGG CTG CGT GT-3'. Denaturation of template DNA was done at 95°C for 2 min, followed by 30 cycles of amplification at 95°C for 30 s, at 63°C for 30 s, and at 72°C for 30 s, and a final extension at 72°C for 5 min. The products were digested with Nla III restriction enzymes at 37°C overnight and identified by agarose electrophoresis. For the individuals with the TT genotype, two bands of fragments (133 bp and 109 bp) were observed. The GT heterozygotes displayed three bands (242 bp, 133 bp, and 109 bp), while the GG homozygotes exhibited a single band (242 bp) [33]. The accuracy of this approach was confirmed through DNA sequencing.
Statistical analysis
The chi-square Goodness of Fit Test was employed to check whether the distribution of the genotypes of TNF-RII rs1061622 was following Hardy-Weinberg Equilibrium. A chi-square test was applied for the analyses of genotype distributions and PTSD prevalence between the male and female participants. The prevalence of PTSD between the subjects with different genotypes of TNF-RII rs1061622 was analyzed by chisquare test, too. Mann-Whitney U test was conducted to analyze PTSD scores of the subjects with different gender, or the subjects with different genotypes of TNF-RII rs1061622. For the identification of severity predictors of PTSD, Stepwise Multiple Linear Regressions were employed. Binary Logistic Analyses were utilized to identify the impact of independent variables on PTSD prevalence. A normal distribution of each variable was determined using the Shapiro-Wilk test initially. TG and TG/HDL-C levels were logarithmically transformed before statistical analyses to reduce skewing. The influence of body mass index (BMI) and age on lipid profiles were adjusted by analysis of covariance (ANCOVA). The value of p≤0.05 was statistically significant.
RESULTS
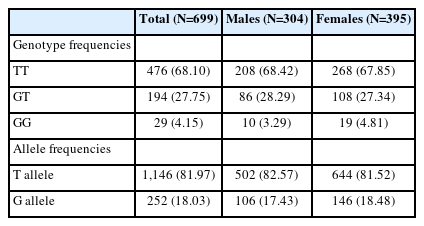
Frequencies of the genotypes and alleles of TNF-RII rs1061622 in the current study population
Table 1 summarizes the frequencies of the genotypes and alleles of TNF-RII rs1061622 in the study population. No deviation was observed from the Hardy-Weinberg Equilibrium in the genotypic distribution of TNF-RII rs1061622 (χ2=2.590, p=0.108). Genotype frequencies of TNF-RII rs1061622 did not show any significant differences between males and females (p=0.600). Due to the insufficient number, GG homozygotes of TNF-RII rs1061622 were taken together with GT heterozygotes and were labeled as GX or G allele carriers for the following analyses.
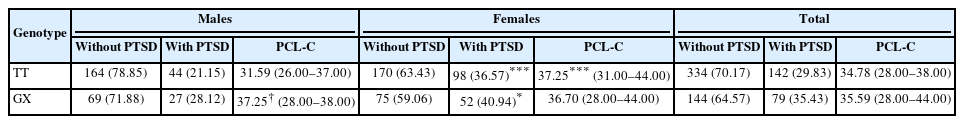
Relationships of TNF-RII rs1061622 with PTSD prevalence and severity
The prevalence and severity of PTSD were investigated among current adolescents with different genotypes of TNF-RII rs1061622 at 6 months after the earthquake in the current study population. As indicated in Table 2, no significant variances of prevalence were noticed between the TT homozygotes and the G allele carriers in all the subjects and the separate sex group. In the other aspect, the female students had higher PTSD prevalence than the male students in both the TT homozygotes (p<0.001) and the G allele carriers (p=0.05).
On the other hand, as presented in Table 2, the G allele carriers had significantly higher PCL-C scores than the TT homozygotes only in the male subjects (p=0.032), but not the female students. In addition, although no significant variances of PCL-C scores were identified between the female G allele carriers and the male G allele carriers, the female TT homozygotes had higher PCL-C scores than the male TT homozygotes (p<0.001).
Relationships between TNF-RII rs1061622, the other factors and PTSD prevalence or severity
To analyze the relationship between TNF-RII rs1061622, the other factors and PTSD prevalence or severity, potential factors associated with PTSD prevalence and predictors of PTSD severity were tested among the subjects with different genotypes of TNF-RII rs1061622 and shown in Table 3.
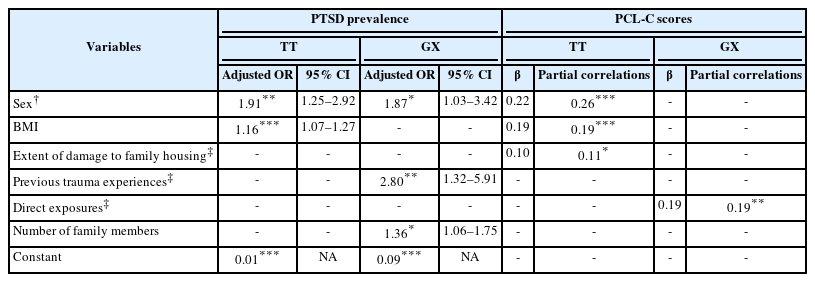
Potential factors associated with PTSD prevalence and predictors of PTSD severity of the subjects with different genotypes of TNF-RII rs1061622
As represented in the table, sex (odds ratio [OR]=1.91, 95% confidence interval [CI]=1.25–2.92, p<0.01) and BMI (OR= 1.16, 95% CI=1.07–1.27, p<0.001) were found to be risk factors for PTSD prevalence in the TT homozygotes. On the other hand, among the G allele carriers, sex (OR=1.87, 95% CI= 1.03–3.42, p=0.041), previous trauma experience (OR=2.80, 95% CI=1.32–5.91, p<0.01), and the number of family members (OR=1.36, 95% CI=1.06–1.75, p=0.015) were potential factors associated with PTSD prevalence. Meanwhile, sex, BMI, and extent of damage to family housing contributed 6.7%, 3.4%, and 1.1%, respectively, of the total variance of PTSD severity in the TT homozygotes, while exposure directly to death or injury of family members or home destruction was the only predictor of PTSD severity, which accounted 3.8% in the G allele carriers.
Relationships between TNF-RII rs1061622 and levels of serum lipids
When exploring the relationships between TNF-RII rs1061622 and serum lipid levels, the influence of BMI and age on lipid profiles was adjusted by ANCOVA. There were no significant differences between the TT homozygotes and the G allele carriers in the whole study population and each sex group. However, the female TT homozygotes had significantly higher TG (p<0.001), TC (p<0.001), HDL-C (p<0.001), and TG/HDL-C (p=0.004) levels than the male TT homozygotes. Similarly, in the G allele subjects, the female subjects also had significantly higher TG (p<0.001), TC (p<0.001), HDL-C (p=0.003), and TG/HDL-C (p=0.004) levels than the male counterparts (Table 4).
Interplays of TNF-RII rs1061622 and PTSD on lipid profiles
When considering the interplays of TNF-RII rs1061622 with PTSD on lipid levels (Table 5), age and BMI were used as covariates because the impacts of these factors were generally recognized on serum lipid levels. The G allele carriers had higher TG (p=0.022) and TG/HDL-C (p=0.019) than the TT homozygotes only in the adolescents without PTSD, but not the subjects with PTSD. Moreover, only the TT homozygotes with PTSD had significantly elevated TC levels (p=0.030) when compared with the TT subjects without PTSD. After considering sexes, the male G allele carriers had higher TG/HDL-C (p=0.045) and TC/HDL-C (p=0.041) than the male TT homozygotes in the subjects without PTSD, while the male G allele carriers had lower TG (p=0.023) and TG/HDL-C (p=0.027) than the male TT homozygotes in the PTSD patients. On the other hand, the female G allele carriers had significantly higher TG (p=0.040) than the female TT homozygotes in the subjects without PTSD. In female PTSD students, no significant differences were found between the TT homozygotes and the G allele carriers.

Effects of PTSD on the association of TNF-RII rs1061622 with anthropometric and biochemical characteristics
No significant differences were found between the male TT homozygotes with and without PTSD after the adjustment for age and BMI when the genotypes were not considered. However, the male G allele carriers with PTSD had lower TG (p=0.016), TG/HDL-C (p=0.001), TC/HDL-C (p=0.031), and higher HDL-C (p=0.021) than the male G allele carriers without PTSD. No significant differences were found between the male TT homozygotes with and without PTSD, and between the female adolescents with and without PTSD in both the TT homozygotes and the G allele carriers. On the other hand, the female TT homozygotes had higher levels of TG (p<0.001), TC (p<0.001), HDL-C (p<0.001), and TG/HDL-C (p=0.002) than the male TT homozygotes in the students without PTSD, while the female TT homozygotes had higher levels of HDL-C (p<0.001) than the male TT homozygotes in PTSD subjects. The female G allele carriers had significantly higher levels of TG (p<0.001), TC (p<0.001), and HDL-C (p<0.001) than the male G allele carriers in the subjects without PTSD. There were higher TG (p<0.001) and TG/HDL-C (p=0.001) in the female G allele carriers than the male G allele carriers in PTSD patients.
DISCUSSION
The pathophysiological and psychopathological mechanism of PTSD, as well as the concomitant metabolic disorders, has not been elucidated yet [34]. Emerging clinical indications suggested that increased soluble TNF-RII (sTNF-RII) was associated with a significantly higher intensity of PTSD and reduced volume of the hippocampus [11]. On the other hand, despite the mutation of TNF-RII rs1061622 could result in the decrease of circulating sTNF-RII levels [35], and was associated with psychological disorders, such as paranoid schizophrenia [22], the lack of previous reports were about the relationship between TNF-RII rs1061622, PTSD, and other factors or the levels of serum lipids. Thus, the present work is the first approach to explore the relationship of PTSD with TNF-RII rs1061622 and other social and psychological factors, and analyze the interplays of PTSD and TNF-RII rs1061622 on lipids levels among adolescent survivors after stressed by the earthquake.
Previous studies indicated that there were no significant differences between male and female subjects of PTSD symptom severity in U.S. PTSD Veterans [36] or in the adult participants from the Millennium Cohort Study [37]. However, the study about Xhosa-speaking high school students in a low-income urban community in South Africa displayed that greater severity of PTSD symptoms was presented in girls when compared with boys [38]. In fact, more evidence can be found in the literature of the inconsistent relationship between PTSD and sex. In the current work, although the prevalence of PTSD was found to be higher in the female subjects than the male counterparts irrespective of the genotypes of TNF-RII rs1061622, the PCL-C scores were significantly higher in the female students than the male subjects only in the TT homozygotes, but not in the G allele carriers (Table 2). In addition, the G allele carriers had significantly increased PCL-C scores when compared with the TT homozygotes only in the female students, but not the male subjects (Table 2). Similarly, sex was found to be a potential factor for PTSD prevalence in either the TT homozygotes or the G allele carriers, but it was observed to be a predictor for PTSD severity only in the subjects with the TT genotype (Table 3). These results suggest interactions among TNF-RII rs1061622, sexes and the other factors, which may have the potential to be a novel explanation for the inconsistent relationship between PTSD and sexes reported by other investigators. More in-depth researches are required for the mechanism involved between TNF-RII rs1061622 and PTSD.
The relationships between PTSD and lipid profiles have been intensively reported. A previous study displayed an increased TC level in combat-related PTSD men when compared with depressive subjects with normal serum lipid profiles [6]. However, no significant changes of serum TC were found in PTSD victims of the Tokyo subway sarin poisoning [9]. More results have been documented that display the discrepancies of relationships between PTSD and lipid profiles.5,6,9 Nevertheless, the mechanism of the discrepancies has not been fully elucidated yet. In our study, although there were no statistically significant differences of lipids levels between the TT homozygotes and the G allele carriers in the study population (Table 4), a significantly higher TC level was found in the subjects with PTSD when compared with those without PTSD only in the TT homozygotes, but not in the G allele carriers (Table 5), suggesting the TNF-RII rs1061622 TT homozygotes might be more sensitive to TC metabolism outcomes resulted from stresses. It is noteworthy that the relationships of PTSD with lipid profiles have not been fully elucidated yet and future studies are needed to explore them.
When sexes were taken into consideration, as indicated in Table 5, elevated TG/HDL-C and TC/HDL-C levels in the G allele carriers than the TT homozygotes were observed in male subjects without PTSD, while the higher level of TG in the G allele carriers than the TT homozygotes was observed in the female students without PTSD. Moreover, decreased levels of TG and TG/HDL-C in the G allele carriers than the TT homozygotes were only found in the male adolescents with PTSD, but not in the male students without PTSD and the female subjects regardless of the status of PTSD. These results suggest interplays between TNF-RII rs1061622 and PTSD on serum lipid profiles in a sex-dependent manner, which may be attributable to the discrepancies of relationships between PTSD and lipid profiles reported before. Besides, Si et al. [39] found that the G allele carriers had elevated levels of TG and TG/HDL-C compared with the TT homozygotes only in the female subjects with suicidal ideation, while the subjects with suicidal ideation had higher TG/HDL-C levels than those without suicidal ideation only in the female G allele carriers. This inconsistency may be caused by different diseases.
Previous studies have indicated a significant decrease of sTNF-RII levels induced by the mutation of TNF-RII rs1061622 [35,40]. Meanwhile, sTNF-RII concentrations were found not only to be positively correlated to TG and TC levels but also to be negatively related to HDL-C levels [41]. Therefore, the changes of serum lipid profiles in the subjects with different genotypes of TNF-RII rs1061622 and different statuses of PTSD may result from the variations of sTNF-RII levels. However, other mechanisms such as linkage disequilibrium and haplotype blockade cannot be excluded currently.
There were some limitations in the current study. First, the levels of sTNF-RII were not tested, although there was still an advantage in the absence of protein checking because the changes of protein levels or structure were not the whole mechanism for the association between gene mutations and phenotypes. Other mechanisms such as linkage disequilibrium and haplotype blockade cannot be excluded currently for the interplays between TNF-RII rs1061622 and PTSD on serum lipid profiles, which were observed in the present study. Second, PTSD was measured by the PCL-C. The PTSD patients recognized in this study might not be the clinical patients. It should be considered when interpreting the results of the present study.
In summary, it is the first study to highlight the association between TNF-RII rs1061622 and PTSD, and their interplay on serum lipids in Chinese adolescents. The results indicated that the G allele carriers presented higher PCL-C scores than the TT homozygotes in the female subjects. The female adolescents manifested higher PCL-C scores than the male subjects in the TT homozygotes. The predictors of PTSD prevalence and severity were observed to be different between the G allele carriers and the TT homozygotes. The subjects with PTSD displayed lower TG, TG/HDL-C, and TC/HDL-C and higher HDL-C than the adolescents without PTSD in the male G allele carriers. The G allele carriers showed higher TG/HDL-C and TC/HDL-C than the TT homozygotes in the male adolescents without PTSD, and lower TG and TG/HDLC in the male PTSD patients. The G allele carriers exhibited higher TG than the TT homozygotes only in the female adolescents without PTSD. These findings demonstrate the possible role of TNF-RII rs1061622 in the prediction of PTSD prevalence and severity, and the potential of the interplays between TNF-RII rs1061622 and PTSD on the levels of serum lipids in a sex-dependent manner, which may be novel explanations for the inconsistent relationship between PTSD and sexes and the discrepancies of relationships between PTSD and lipid profiles, provide novel insights into the development of PTSD and the regulation of serum lipids, and pave the way for precision medicine interferences of PTSD and comorbidities of PTSD and hyperlipidemia among adolescents with different genetic backgrounds of TNF-RII rs1061622 after experiencing traumatic events.
Notes
Availability of Data and Material
The datasets used and/or analyzed during the current study are not publicly available due to the information that could compromise the participant’s privacy and the agreement with the school, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Ding Zhi Fang. Data curation: Ji Cheng Zhang, Jin Hua Wang, Jun Yi Liu, Ke Xin Jia, Jia Jing Cai, Guo Ming Su. Formal analysis: Ji Cheng Zhang, Jin Hua Wang, Qi Wei Guo, Yi Lin Shen. Funding acquisition: Ding Zhi Fang. Investigation: Ji Cheng Zhang, Jin Hua Wang, Jun Yi Liu, Qi Wei Guo, Jia Lin, Yi Lin Shen, Ke Xin Jia, Jia Jing Cai, Guo Ming Su. Methodology: Ji Cheng Zhang, Jin Hua Wang, Ding Zhi Fang. Project administration: Jia Lin. Resources: Jia Lin. Supervision: Ding Zhi Fang. Validation: Ding Zhi Fang. Visualization: Ji Cheng Zhang, Jin Hua Wang. Writing—original draft: Ji Cheng Zhang, Jin Hua Wang. Writing—review & editing: Ding Zhi Fang.
Funding Statement
This work was supported by the Major Project of Sichuan for Science and Technology (Grant No. 2022YFH0025). Professor Ding Zhi Fang is the recipient of the grant.