 |
 |
- Search
| Psychiatry Investig > Volume 19(2); 2022 > Article |
|
Abstract
Objective
Methods
Results
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding authors upon reasonable request. For access and sharing, biological resources of the BICWALZS were stored in the BICWALZS consortium biobank (www.bicwalzs.com/) as well as the National Biobank of Korea (www.nih.go.kr/biobank) which is a central biobank supporting for the Korea Biobank Project.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Sang Joon Son, Chang Hyung Hong. Data curation: Hyun Woong Roh, Na-Rae Kim, Dong-gi Lee. Formal analysis: Hyun Woong Roh, Na-Rae Kim, Dong-gi Lee. Investigation: Hyun Woong Roh, Jae-Youn Cheong, Sang Won Seo, Seong Hye Choi, Eun-Joo Kim, Soo Hyun Cho, Byeong C. Kim, Seong Yoon Kim, Sang Joon Son, Chang Hyung Hong. Methodology: Eun Young Kim, Jaerak Chang, Sang Yoon Lee, Dukyong Yoon, Jin Wook Choi, Young-Sil An, Hee Young Kang, Hyunjung Shin, Bumhee Park. Resources: Sang Joon Son, Chang Hyung Hong. Software: Hyun Woong Roh, Na-Rae Kim, Bumhee Park. Supervision: Sang Joon Son, Chang Hyung Hong. Validation: Sang Joon Son, Chang Hyung Hong. Visualization: Hyun Woong Roh, Na-Rae Kim. Writing—original draft: Hyun Woong Roh, Na-Rae Kim. Writing—review & editing: Bumhee Park, Sang Joon Son, Chang Hyung Hong.
Funding Statement
This study was conducted with biospecimens and data from the consortium of the Biobank Innovations for Chronic Cerebrovascular Disease with ALZheimer’s Disease Study (BICWALZS), which was funded by the Korea Disease Control and Prevention Agency for the Korea Biobank Project (#4845-303). This work was also supported and funded by the grant from National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2019R1A5A2026045) and the grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), and the Ministry of Health & Welfare, Republic of Korea (grant number: HR21C1003).
Figure 1.
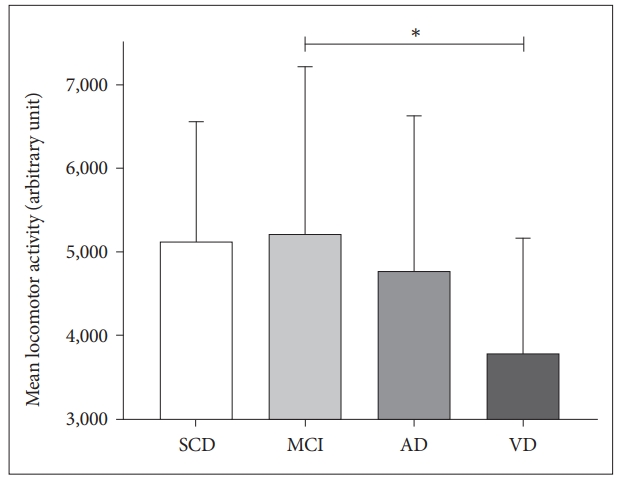
Figure 2.
Table 1.
| Participant characteristics | SCD (N=168) | MCI (N=534) | AD (N=211) | VD (N=80) | Others (N=20) | p† | p<0.05‡ | |
|---|---|---|---|---|---|---|---|---|
| Age, years | 70.9±7.1 | 72.6±6.9 | 74.1±7.8 | 75.1±7.0 | 68.9±8.1 | <0.001 | a, b, c, d, e | |
| Female | 115 (68.5) | 356 (66.7) | 127 (60.2) | 50 (62.5) | 10 (50.0) | 0.274 | ||
| Education, years | 7.7±4.0 | 8.0±4.9 | 8.5±5.0 | 6.4±4.7 | 8.8±4.6 | 0.013 | e, f | |
| Hypertension | 99 (58.9) | 282 (52.8) | 122 (57.8) | 54 (67.5) | 8 (40.0) | 0.061 | ||
| Diabetes | 36 (21.4) | 111 (20.8) | 57 (27.0) | 37 (46.3) | 3 (15.0) | <0.001 | ||
| Dyslipidemia | 66 (39.3) | 202 (37.8) | 58 (27.5) | 29 (36.3) | 5 (25.0) | 0.042 | ||
| APOE e4 carrier | 26 (15.5) | 133 (24.9) | 98 (46.5) | 17 (21.3) | 4 (20.0) | <0.001 | ||
| MMSE (0-30) | 26.2±2.8 | 24.2±3.8 | 19.0±5.1 | 18.2±4.8 | 20.6±5.3 | <0.001 | a, b, c, d, e | |
| S-IADL | 3.4±3.6 | 6.6±5.6 | 16.4±10.4 | 19.7±11.1 | 19.1±9.2 | <0.001 | a, b, c, d, e, f | |
| SGDS-K | 4.3±4.0 | 6.6±5.0 | 5.4±4.6 | 6.9±5.1 | 9.9±4.1 | <0.001 | a, c, d | |
| GDS | 2.3±0.5 | 3.0±0.7 | 4.2±0.9 | 4.3±0.9 | 4.4±1.1 | <0.001 | ||
| Aβ deposition on PET | N=82 | N=400 | N=152 | N=62 | N=17 | <0.001 | ||
| (N=713) | 7 (8.5) | 107 (26.8) | 129 (84.9) | 9 (14.5) | 2 (11.8) | |||
| Schelten’s scale | 1.2±0.8 | 1.6±0.8 | 2.3±0.9 | 2.6±0.8 | 1.9±0.7 | <0.001 | a, b, c, d, e, f | |
| WMH on MRI (N=817) | N=102 | N=441 | N=182 | N=73 | N=19 | <0.001 | ||
| Minimal | 73 ±71.6 | 283 (64.2) | 94 (51.7) | 10 (13.7) | 16 (84.2) | |||
| Moderate | 25 (24.5) | 139 (31.5) | 74 (40.7) | 33 (45.2) | 3 (15.8) | |||
| Severe | 4 (3.9) | 19 (4.3) | 14 (7.7) | 30 (41.1) | 0 (0.0) | |||
Data are shown as the mean±SD or number (%).
AD; c, SDC vs. VD; d, MCI vs. AD; e, MCI vs. VD; f, AD vs. VD. SCD, subjective cognitive decline; MCI, mild cognitive impairment; AD, Alzheimer’s dementia; VD, vascular dementia; APOE, apolipoprotein E; MMSE, mini-mental state examination; S-IADL, Seoul instrumental activities of daily living; SGDS-K, Korean version of the short form of geriatric depression scale; GDS, global deterioration scale; WMH, white matter hyperintensities
Table 2.
| Neuropsychological test | SCD (N=168) | MCI (N=534) | AD (N=211) | VD (N=80) | Others (N=20) | p† | p<0.05‡ | |
|---|---|---|---|---|---|---|---|---|
| Attention function | ||||||||
| Digit Span Test-backward | -0.14 (1.11) | -0.51 (1.09) | -1.09 (1.29) | -1.27 (1.23) | -1.38 (1.40) | <0.001 | a, b, c, d, e | |
| Language function | ||||||||
| Boston naming test | 0.40 (0.85) | -0.47 (1.32) | -1.61 (1.65) | -1.60 (1.97) | -2.35 (2.18) | <0.001 | a, b, c, d, e | |
| Visuospatioal function | ||||||||
| RCFT-copy | -0.04 (1.07) | -0.70 (4.70) | -2.34 (2.48) | -2.50 (2.02) | -3.38 (3.07) | <0.001 | b, c, d, e | |
| Memory function | ||||||||
| SVLT-delayed recall | 0.21 (0.95) | -0.89 (1.08) | -1.84 (0.94) | -1.64 (0.86) | -1.88 (0.72) | <0.001 | a, b, c, d, e | |
| RCFT-delayed recall | 0.13 (0.98) | -0.73 (0.96) | -1.59 (0.78) | -1.16 (0.80) | -1.12 (1.13) | <0.001 | a, b, c, d, e, f | |
| Frontal/executive function | ||||||||
| Stroop test-color reading | 0.09 (0.95) | -0.77 (1.28) | -1.82 (1.63) | -2.10 (1.24) | -2.74 (1.53) | <0.001 | a, b, c, d, e | |
| COWAT-phonemic task | 0.06 (0.89) | -0.64 (0.92) | -1.36 (1.05) | -1.58 (0.73) | -1.56 (0.83) | <0.001 | a, b, c, d, e | |
a, SCD vs. MCI; b, SCD vs. AD; c, SDC vs. VD; d, MCI vs. AD; e, MCI vs. VD; f, AD vs. VD. AD, Alzheimer’s dementia; MCI, mild cognitive impairment; SCD, subjective cognitive decline; VD, vascular dementia; RCFT, Rey complex figure test; SVLT, Seoul verbal learning test; COWAT, controlled oral word association test-phonemic task
REFERENCES







