|
 |
- Search
| Psychiatry Investig > Volume 18(8); 2021 > Article |
|
Abstract
Objective
Methods
Results
Supplementary Materials
Supplementary┬ĀTable┬Ā2.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Shiping Xie, Min Zhou. Data curation: Wenwen Xu, Wenying Ma. Formal analysis: Xiaolei Qiu. Funding acquisition: Shiping Xie, Min Zhou. Investigation: all authors. Methodology: Xiaolei Qiu, Rongrong Zhang. Project administration: Shiping Xie, Min Zhou. Resources: Rongrong Zhang, Wei Yan. Software: Xiaolei Qiu, Wenying Ma. Supervision: Shiping Xie. Validation: Shiping Xie, Xiaolei Qiu, Wenwen Xu. Visualization: Xiaolei Qiu. WritingŌĆöoriginal draft: Xiaolei Qiu. WritingŌĆöreview & editing: Xiaolei Qiu, Rongrong Zhang, Wei Yan, Shiping Xie, Min Zhou.
Funding Statement
This study was partially supported by the General Program of Jiangsu commission of health (H2017051), and the Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306805).
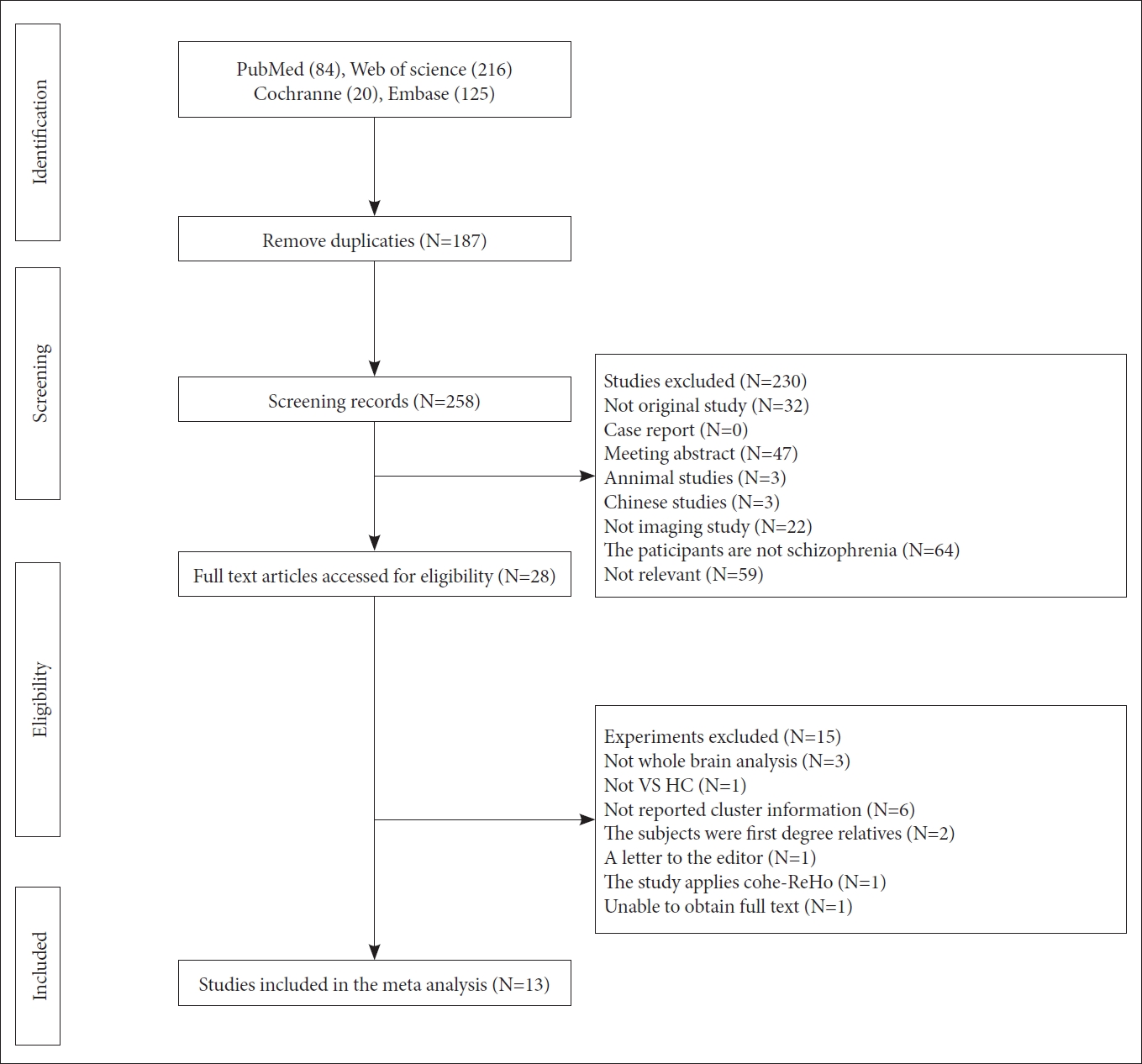
Figure┬Ā2.
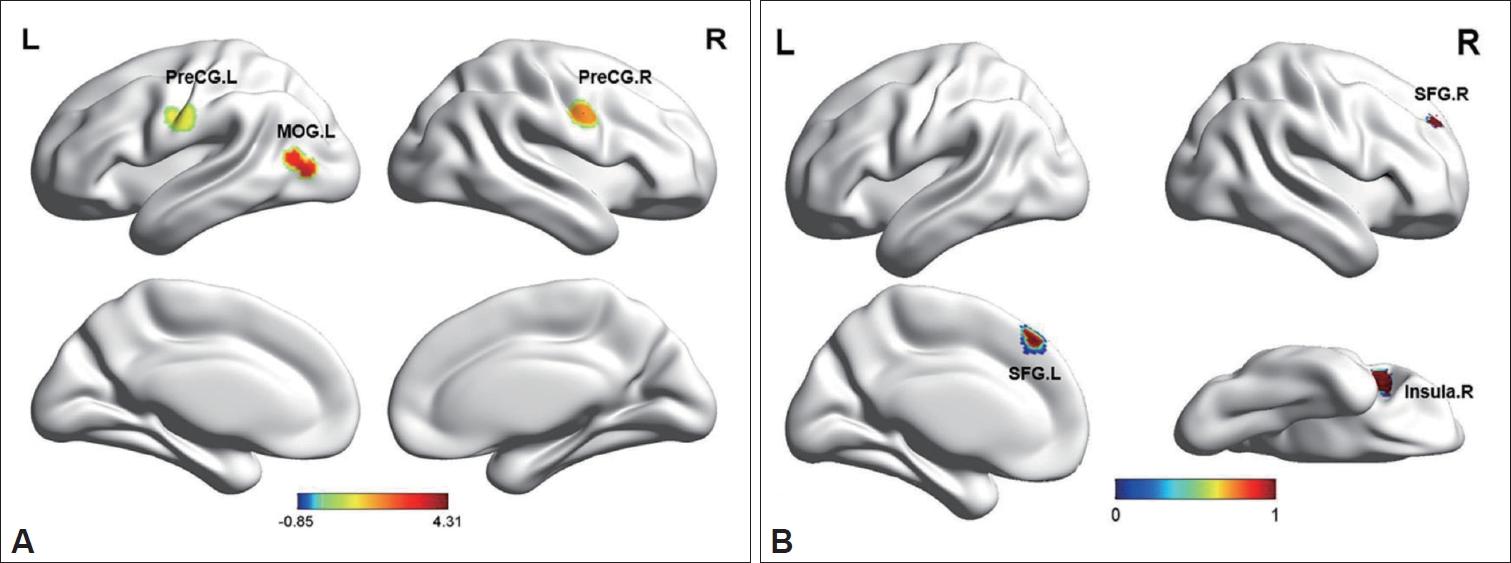
Figure┬Ā3.
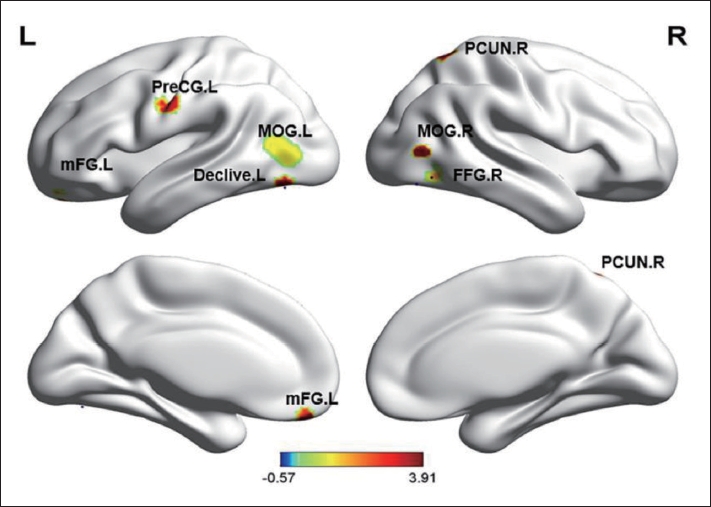
Table┬Ā1.
| Author | Sample size | Sex (M/F) | Diagnostic criteria | SZ age (mean┬▒SD) | HC age (mean┬▒SD) | Diagnosis | Drug state | Quanlity score |
|---|---|---|---|---|---|---|---|---|
| Shan et al. [22] | SZ 39 | 27/12 | DSM-5 | 24.64┬▒5.01 | 25.7┬▒4.9 | SZ | Yes | 9.5 |
| HC 20 | 14/6 | |||||||
| Zhao et al. [23] | SZ 44 | 31/13 | DSM-IV | 23.7┬▒5.3 | 22.6┬▒3.7 | FES | Drug naive | 10 |
| HC 26 | 17/9 | |||||||
| Zhang et al. [24] | SZ 53 | 42/11 | DSM-IV | 36.7┬▒13.67 | 34.82┬▒11.28 | SZ | Yes | 9.5 |
| HC 67 | 46/21 | |||||||
| Wang et al. [8] | SZ 48 | 21/27 | DSM-IV-TR | 15.79┬▒1.64 | 15.42┬▒1.52 | FES | Drug naive | 10 |
| HC 31 | 14/17 | |||||||
| Gou et al. [25] | SZ 28 | 16/12 | DSM-IV | 23.9┬▒5.4 | 28.8┬▒6.1 | SZ | Yes | 9.5 |
| HC 21 | 14/7 | |||||||
| Gao et al. [27] | SZ 34 | 19/15 | DSM-IV | 35.13┬▒8.85 | 32.73┬▒7.61 | SZ | Yes | 10 |
| HC 29 | 16/13 | |||||||
| Liu et al. [11] | SZ 27 | 15/12 | DSM-IV | 25.44┬▒5.92 | 27.44┬▒7.24 | SZ | Partly | 9.5 |
| HC 27 | 18/9 | |||||||
| Cui et al. [28] | SZ 32 | NA | DSM-IV-TR | NA | NA | FES | Drug naive | 9 |
| HC 19 | ||||||||
| Gao et al. [26] | SZ 14 | 9/5 | DSM-IV | 33.2┬▒10.7 | 34.9┬▒113.6 | SZ | Yes | 9.5 |
| HC 14 | 9/5 | |||||||
| Yu et al. [9] | SZ 69 | 34/35 | DSM-IV | 31.7┬▒9.6 | 29.9┬▒8.6 | SZ | Yes | 9.5 |
| HC 62 | 25/27 | |||||||
| Liu et al. [12] | SZ 18 | 9/9 | DSM-IV | 23.67┬▒4.397 | 24.44┬▒3.884 | SZ | Yes | 9 |
| HC 18 | 9/9 | |||||||
| Chen et al. [30] | SZ 36 | 16/20 | DSM-IV | 28.28┬▒1.43 | 35.68┬▒1.81 | SZ | Yes | 9 |
| HC 44 | 17/27 | |||||||
| Yan et al. [29] | SZ 69 | 50/19 | DSM-IV | 24.22┬▒7.08 | 26.27┬▒6.97 | FES | Drug naive | 10 |
| HC 74 | 45/29 |
Table┬Ā2.
| Author | Scanners | Eye state | Repetition time/echo time | Thickness (mm) | Slice gap (mm) | FWHM (mm) | Threshold | Coordinate |
|---|---|---|---|---|---|---|---|---|
| Shan et al. [22] | 3.0T | Closed | 2,000/30 ms | 4 | 0.6 | 4 | Corrected | MNI |
| Zhao et al. [23] | 3.0T | Closed | 2,000/30 ms | 4 | 0.5 | 4 | Corrected | MNI |
| Zhang et al. [24] | 3.0T | Closed | 2,000/29 ms | 3 | NA | 6 | Corrected | MNI |
| Wang et al. [8] | 3.0T | NA | 2,000/30 ms | 4 | 0.6 | 4 | Corrected | MNI |
| Gou et al. [25] | 1.5T | Closed | 2,000/40 ms | 5 | 1 | 4 | Corrected | MNI |
| Gao et al. [27] | 3.0T | NA | 2,000/30 ms | 4 | 0.6 | 4 | Corrected | MNI |
| Liu et al. [11] | 1.5T | NA | 2,000/40 ms | 5 | 1 | 8 | Uncorrected | MNI |
| Cui et al. [28] | 3.0T | Closed | 2,000/30 ms | 4 | 0.6 | 4 | Corrected | MNI |
| Gao et al. [26] | 1.5T | Closed | 2,000/40 ms | 5 | 1 | 6 | Corrected | MNI |
| Yu et al. [9] | 3.0T | NA | 2,000/24 ms | 3 | 0 | 6 | Corrected | MNI |
| Liu et al. [12] | 1.5T | NA | 2,000/40 ms | 5 | 1 | 4 | Corrected | MNI |
| Chen et al. [30] | 3.0T | Closed | 580/18 ms | 4 | 0 | 4 | Corrected | Talairach |
| Yan et al. [29] | 3.0T | Closed | 2,500/30 ms | 3.5 | 1 | 4 | Corrected | MNI |
Table┬Ā3.
Table┬Ā4.
REFERENCES