|
 |
- Search
| Psychiatry Investig > Volume 17(3); 2020 > Article |
|
Abstract
Objective
This study investigates the association between mental disorders and interferon nontreatment in patients with chronic hepatitis C virus (HCV) infection in a large national sample.
Methods
Using the National Health Insurance Research Database of Taiwan, we conducted a nationwide population-based study. Each case was matched to five controls by age, sex, urbanization, and income. Conditional logistic regression was used to assess odds of HCV nontreatment in different mental disorders.
Results
From 1999 to 2013, we identified 92,970 subjects with HCV infection and 15,495 HCV cases (16.7%) had received IFN therapy. Other than chronic obstructive pulmonary disease, the medical diseases and mental disorders were significantly different between IFN and non-IFN treated HCV patients. After adjusting for medical diseases, depressive disorder and anxiety disorder was positively associated with receiving IFN therapy. Patients with schizophrenia, bipolar disorders and alcohol use disorders were significantly less likely to receive interferon. Antidepressant exposure (cumulative daily exposure or cumulative daily dose) was associated with lower odds of IFN treatment.
Conclusion
Our nationwide cohort study demonstrated that INF nontreatment rate was lower in certain mental disorders. Antidepressant exposure might lower the chance of receiving IFN treatment. Our results may help to identify and to overcome the obstacles for HCV treatment and further apply to DAAs regimen.
Hepatitis C virus (HCV) infection is a major global health problem. More than 170 million people, 2.2% of adult worldwide, are chronically infected with HCV [1,2]. Previous studies have shown that 60-85% of HCV infected patients may develop chronic infection [3]. Chronic HCV infection often leads to progressive liver fibrosis, end-stage liver disease and hepatocellular carcinoma (HCC). The risk whether a person with chronic hepatitis C after 20 years will develop HCC appears to be around 1-5% [4]. Once liver cirrhosis occurs, the annual rate of developing HCC increases by 1-4% [5]. In Taiwan, the estimated prevalence of HCV infection is 1-3% [6-8]. HCV prevalence in several southern Taiwan townships may even reach 15% [9,10]. In addition, a pathogenic role for HCV in neuropsychiatric disorders and neurocognitive dysfunction is suggested by improvement of neurological and psychiatric symptoms in patients achieving a sustained virologic response following interferon treatment [11]. Hence, effective management of chronic HCV infection to abate further deterioration is very crucial.
Interferon (IFN)-based therapy had been the standard care of HCV infection. Since 2011, the U.S. Food and Drug Administration has approved several direct antiviral agents (DAAs) for the treatment of HCV. However, the first-generation DAAs were first approved in Taiwan until 2014 and were not reimbursed by health insurance. The high costs of these new regimens preclude their widespread use. Therefore, the long-term complications and HCC risk of DAAs on HCV patients need more observation. The combination of pegylated IFN and ribavirin are more commonly used and the medications can eradicate the virus in more than 50% of patients [12]. However, only a minority of people receive anti-HCV treatment. Treatment rate in patients with HCV infection ranged from 15-28% in different studies according to patient selection, illness comorbidity, or ethics [13-16]. Because of high rates of psychiatric disorders among patients with HCV, one often cited barrier to antiviral treatment is co-morbid mental illness [17,18]. The prevalence of HCV infection in psychiatric populations has been found to be around 6.7% to 8.5%, which is 3-fold higher than in the general population [19]. About a quarter of HCV patients meet DSM-IV criteria for major depressive disorder, while 45-60% of HCV patients have mild to severe depressive symptoms [16]. On the other way, depression and other psychiatric symptoms are well-known side effects of IFN therapy and often deters clinicians from prescribing IFN in HCV-infected people [20].
Patients with mental disorders are disproportionately affected by HCV and are less likely to undergo HCV treatment. The nontreatment rate of INF in psychiatric populations was reported to be around 7.2-10.1% [21,22]. In a large national veterans population, treatment rate for hepatitis C was significant lower in patients with major depression (OR 0.72; 95% CI 0.67 to 0.77); mild depression (OR 0.56; 95% CI 0.53 to 0.59); and schizophrenia (OR 0.71; 95% CI 0.65 to 0.77) [18]. However, large clinical trials examining the efficacy and tolerability of IFN-a/ribavirin have largely excluded patients with pre- existing psychiatric disease out of concern for neuropsychiatric sequelae [20]. There is still a lack of large-scale research that studies the IFN prescription rate in patients comorbid with hepatitis C and mental disorder and study duration was relatively limited. Hence, this population-based cohort study aimed to investigate the association between comorbid mental disorder and the rates of IFN nontreatment in HCV patients using data from the National Health Insurance (NHI) of Taiwan.
This study retrieved data from the Taiwan National Health Insurance Research Database (NHIRD). The NHI program was launched in Taiwan on 1 March, 1995. More than 23.07 million people were enrolled in the program by the end of 2013, and it covered 99.6% of the entire population of 23.16 million of Taiwan [23]. Starting in 1999, all claims data was released in electronic format by the Bureau of NHI (NHIB) and was made publicly available under the NHIRD project. The database provides scrambled patient identification numbers, gender, dates of birth, and diagnoses according to the format of the International Classification of Diseases, Revision 9, Clinical Modification (ICD-9-CM), prescribed medication, medical costs, medical care facilities and their specialties. We retrieved medical data from enrolled individuals between January 1st, 1999, and December 31th, 2013. These databases have previously been used for epidemiological research, and were published [24,25]. Following strict confidentiality guidelines in accordance with personal electronic data protection regulations, the NHRI of Taiwan maintains an anonymous database of NHI reimbursement data suitable for research.
All cases of HCV had a first-time diagnosis of HCV infection (ICD-9 codes 070.7, 070.41, 070.44, 070.51, 070.54, V02.62). Patients with hepatitis B virus (HBV) infection (ICD-9 codes 070.2, 070.3, V02.61) or HIV infection (ICD-9 code 042) were excluded.
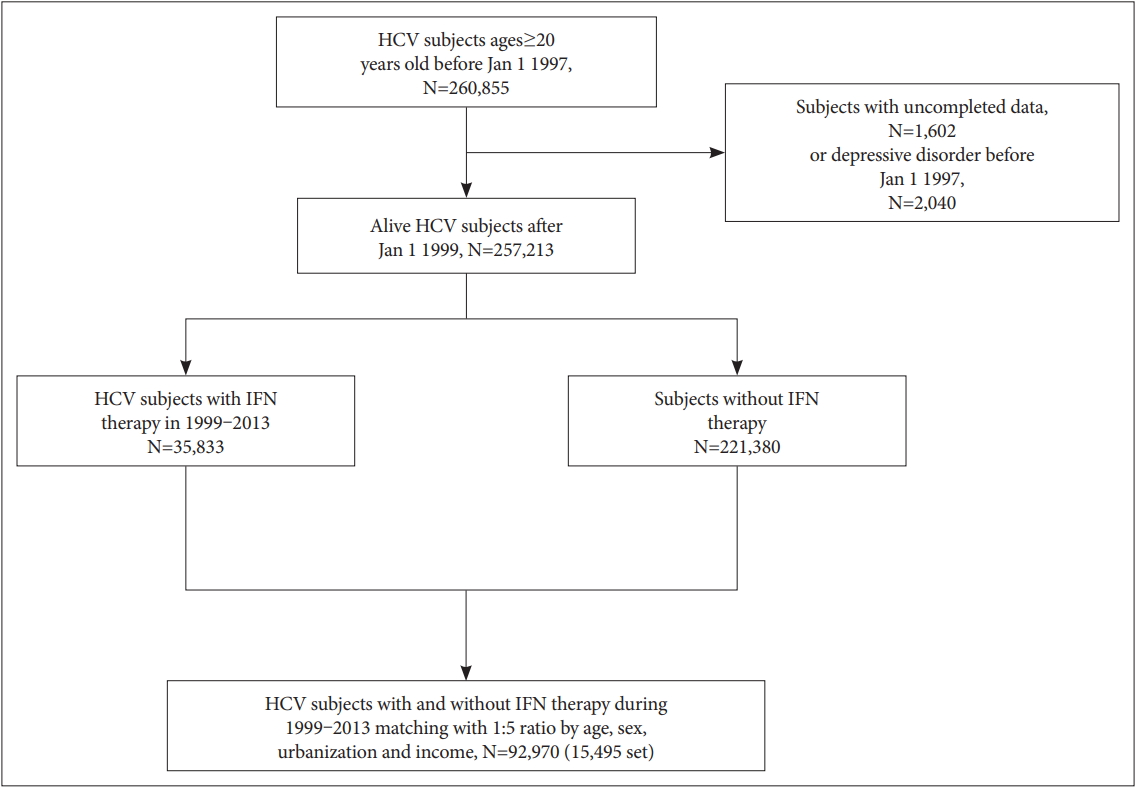
We assembled a population-based matched case-control study comprised of individuals who have been participants of the NHI program since January 1, 1997. The study outcome was incidence of IFN-α therapy (ATC codes L03AB04, L03AB05, L03AB09, L03AB10, or L03AB11). IFN-α therapy in the HCV cohort was denoted if the patient had any exposure to IFN-α therapy. To be included in the control population, the HCV infected individual was required to have no IFN-α therapy before the index date. For each IFN-α treated case, incidence density sampling method was used [26] and 5 matched controls were randomly selected at index date. Controls were age, sex, urbanization and income-matched to the case (Figure 1).
The following mental disorders were recorded, including psychotic disorders (ICD-9 codes 296.80, 296.9, 297, 298), schizophrenia (ICD-9 code 295), bipolar disorders (ICD-9 codes 296.0, 296.1, 296.4, 296.5, 296.6, 296.7), depressive disorders (ICD-9 codes 296.2, 296.3, 300.4, 311), anxiety disorders (ICD-9 codes 300.0, 300.2, 300.3) and alcohol use disorders (ICD-9 codes 303.9, 303.90, 303.91, 303.92, 303.93, 305.0, 305.00, 305.01, 305.02, 305.03, V113). All diagnosed subjects had at least three outpatient visits within one year or one incident recorded with the admission diagnosis during the follow-up. We used the pharmacological coding system based on the Anatomical Therapeutic Chemical classification system [27] to categorize antidepressants (N06A). Antidepressant exposure was standardized using the defined daily dose (DDD) [27]. Cumulative doses were classified into 4 groups: no antidepressant use; 1-90 DDD; 91-365 DDD; greater than 365 DDD.
We systematically identified potential confounding factors for IFN-α treatment. These confounding factors were recorded before the date of IFN-α therapy: age, sex, income, residential area (urbanization), medical comorbidity, and drug use (antidepressants and zolpidem). Levels of income and urbanization in Taiwan were divided into four strata according to Taiwan NHRI publications. Comorbid medical diseases included hypertension, diabetes, hypercholesterolemia, chronic obstructive pulmonary disease (COPD), heart failure, coronary heart disease, and liver cirrhosis. The study was approved by the Institutional Review Board of Chiayi Chang Gung Memorial Hospital (IRB No. 2017-00253B0).
Subjects’ age, sex and clinical confounding factors and covariates were examined using χ2 test for categorical variables and Student t-test for continuous variables. All statistical tests were two sided, conducted at significance level of 0.05, and reported using p value and/or 95% confidence intervals (CIs).
Conditional logistic regression was used to assess odds of HCV nontreatment in different mental disorder. The odds ratios (ORs) and 95% Cls for HCV nontreatment were calculated as an unadjusted incidence rate ratio, and then subsequently adjusted for covariates (medical comorbidities). All analyses were conducted using the SAS Version 9.4 (SAS Institute, Cary, NC, USA).
We identified 92,970 subjects with hepatitis C virus infection from 1999 to 2013. Among them, 15,495 cases (16.7%) had received IFN therapy, and 77,475 cases (83.3%) had not. Sociodemographic characteristics including age, income and urbanization, comorbid medical diseases and mental disorders are listed in Table 1. The mean age of subjects was 40.95±10.31 for both groups (p=0.995). More than half (56.72 %) of the cases and controls were female. IFN treated patients were more likely to have a diagnosis of diabetes, hypertension, liver cirrhosis, depressive disorder, and anxiety disorders (Table 1), and less likely to have hypercholesterolemia, heart failure, coronary heart disease, alcohol use, psychotic disorders, schizophrenia, and bipolar disorders (Table 1). IFN treated and nontreated patients showed no difference in COPD. Analyses of associations of interest between mental disorders and IFN therapy are summarized in Table 2. After adjusting for medical diseases, depressive disorders and anxiety disorders were positively associated with receiving IFN therapy (OR 1.08, 95% CI: 1.02-1.14, p=0.01; OR 1.16, 95% CI: 1.10-1.21, p<0.0001, respectively). Patients with schizophrenia, bipolar disorders and alcohol use disorder were less likely to receive interferon, and the association remained unchanged even after adjusting for covariates (Table 2). Because the severity of depressive disorders was not recorded in NHIRD, we analyzed antidepressant prescription as a proxy of severity of depressive disorder. Cumulative daily exposure to antidepressants had a negative association with IFN therapy (1-90 cDays, adjusted OR=0.92, 95% CI=0.87-0.96; 91-365 cDays, adjusted OR= 0.93, 95% CI=0.85-1.01; >365 cDays, adjusted OR=0.79, 95% CI=0.72-0.86). Likewise, cumulative use of antidepressants (cDDD) was associated with lower odds of IFN therapy (1-90 cDDD, adjusted OR=0.92, 95% CI=0.88-0.96; 91-365 cDDD, adjusted OR=0.87, 95% CI=0.78-0.96; >365 cDDD, adjusted OR=0.77, 95% CI=0.69-0.86) (Table 3). Dose-dependent trend in antidepressant exposure of cumulative days or doses was observed between the case and control populations (p<0.0001).
To the best of our knowledge, this is the first Asian population-based study to explore the association between mental disorders and HCV nontreatment in terms of interferon use. Although DAAs are the mainstream treatment of HCV infection in recent years [28], sustained virologic response has remained low at approximately 9% of all HCV infected persons [29]. Baseline mental conditions might compromise the patient’s or the clinician’s motive to receiving or giving DAAs regimen. How to facilitate treatment engagement and adherence to DAAs is paramount during HCV treatment. We can learn from the experiences of IFN non-treatment and further apply them in post-IFN era to enhance drug adherence.
We found that 13.9% (35,833/257,213) of HCV-infected subjects were prescribed IFN between years 1999 and 2013. This is in line with Kramer’s study that the overall treatment rate was 14.2% and the treatment rate would be even lower if the HCV patients had comorbid physical diseases or mental disorders [30]. In our study, after matching with age, sex, residential areas, and income level, the medical diseases and mental disorders were significantly different between IFN-treated and non-treated patients. HCV patients with diabetes, hypertension, liver cirrhosis, depressive disorders and anxiety disorders were less likely to receive IFN treatment. Patients with hypercholesterolemia, heart failure, coronary heart disease, alcohol use, psychotic disorders, schizophrenia, and bipolar disorders were associated with a higher chance of receiving IFN treatment. Butt et al. [18] reported several medical diseases such as decompensated cirrhosis, heart and kidney failure often accounts for a significant proportion of IFN nontreatment. The medical comorbidities may deter the HCV-infected patients from receiving treatment.
In the logistic regression model, we found schizophrenia, bipolar disorders, and alcohol use disorders were associated with a lower likelihood of IFN prescription after adjusting for medical diseases. This is consistent with other studies that HCV patients with some mental disorders or substance use were treated less frequently [31,32]. However, previous reports mainly focused on veterans or non-national population and their sample size was small and study duration was relatively limited. Chainuvati et al. [31] reported that a significantly lower proportion of patients with acute mental illness or substance abuse received IFN treatment because of ineligibility. However, treatment completion and sustained viral response did not differ significantly between patients with or without mental illness/ substance abuse. This implies that patients whose mental illnesses are under control and who are regularly followed by mental health providers are often good candidates for antiviral therapy. Hence, these patients should not be excluded from receiving IFN treatment.
In our study, patients with depressive or anxiety disorders were more likely to receive IFN, this phenomenon may reflect patient’s suffering or physician’s preference. Unlike our study, Butt et al. [18] reported that mental disorders, including bipolar disorder, major depression, mild depression, and schizophrenia, were inversely related to HCV treatment. Patients with both depression and HCV often perceive lower social support and greater social stigma about their conditions. These beliefs may enhance the perception that there is a lack of providers willing to treat or interested in treating HCV patients [33]. In addition to patients’ comorbid illness, an important factor in IFN nontreatment was explained by provider factors [34]. Patients evaluated by less experienced providers were less likely to receive IFN treatment. Physicians may be hesitant in prescribing IFN in such patients because depression is a common event of IFN treatment [20], and is a main worry when physicians make treatment decisions in HCV-infected people.
Because disease severity was not included in NHIRD of Taiwan, we further looked at the antidepressant exposure in terms of duration and dosage as a proxy of depressive severity. Antidepressant exposure was inversely related to IFN treatment. Dose-dependent effect was shown when patients had received more antidepressants (cumulative days or doses), the odds of IFN treatment rate was significantly lower. This elucidates that depression severity may pose a barrier to HCV treatment. Two studies also found that higher depressive scores were independently associated with IFN nontreatment after controlling for sociodemographic factors [17,19]. Nelligan et al. [35] found that HCVinfected veterans patients endorsing moderate to severe depressive symptoms, only about half were prescribed an antidepressant. HCV patients with more depressive symptoms at baseline were more likely to develop clinically significant depression during IFN treatment. It is well-documented that depression is associated with poor self-efficacy, reduced motivation, greater perceived stigmatization, pessimism and reluctance to seek healthcare services [36,37]. Depression reduces patient’s motivation to seek medical care, consequently these patients may remain untreated because of their depressive symptoms [17]. Furthermore, patients with depression and HCV may go untreated because many patients with HCV are concerned about taking any medications that may jeopardize their liver health including antidepressants.
Our study had some limitations. First, the NHRID files did not include records of other factors including HCV genotype, pretreatment HCV RNA level, histology finding, and severity of psychiatric illness. Such factors may be related to IFN prescription. Second, only the clinical diagnosis of mental disorder was retrieved and biomarker was not included for diagnosis in the NHIRD. In order to validate the psychiatric diagnosis, we used three outpatient visit records or one inpatient record to decrease selection bias. Finally, the study sample was in Taiwan; the results might not be generalized to other populations. Future research examining these confounding factors and IFN treatment with larger sample sizes and longer follow-up periods is required.
Despite these limitations, this study has several strengths. The data were extracted from a nationwide database spanning 14 years, the sample size was large and selection bias is minimized. Confounding factors such as income, urbanization, and medical comorbidities were adjusted for in the analysis. Diagnostic and prescription data were retrieved from a historical electronic database that collected all medical claims, the possibility of recall bias can be minimized. In our study, we excluded patients who were co-infected with HBV or HIV, this further elucidated the reasons why HCV patients had not received IFN treatment.
Our nationwide cohort study demonstrated that INF nontreatment rate was lower in patients with psychotic disorders, schizophrenia, and bipolar disorders but not anxiety disorders and depressive disorders. Dose-dependent effect was found that more antidepressant exposure may lower the chance of receiving IFN treatment. Our results may help to identify and to overcome the obstacles to HCV treatment and further apply to DAAs regimen.
ACKNOWLEDGEMENTS
The authors would like to thank the Health Information and Epidemiology Laboratory (CLRPG6G0041) for their comments and assistance in data analysis. This study was supported by a grant from Chang Gung Memorial Hospital, Chia-Yi Branch, and based on the National Health Insurance Research Database provided by the Central Bureau of National Health Insurance, the Department of Health, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
The study is supported in part by the Ministry of Science and Technology, Taiwan, ROC (MOST 102-2314-B-040-004-MY3) and Chang Gung Medical Foundation, Chiayi Chang Gung Memorial Hospital, Taiwan, ROC (CMRPG6E0261).
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Wei-Che Chiu, Cheng-Chen Chen. Data curation: Wei-Che Chiu, Mong-Liang Lu. Formal analysis: Wei-Che Chiu. Funding acquisition: Cheng-Chen Chen. Investigation: Wei-Che Chiu, ChengChen Chen. Methodology: Wei-Che Chiu. Project administration: ChengChen Chen. Resources: Cheng-Chen Chen. Software: Wei-Che Chiu. Supervision: Mong-Liang Lu, Cheng-Chen Chen. Validation: Wei-Che Chiu, Cheng-Chen Chen. Writing—original draft: Wei-Che Chiu. Writing—review & editing: Mong-Liang Lu, Cheng-Chen Chen.
Table 1.
Characteristics of subjects
Table 2.
Association between mental disorders and IFN treatment in a population-based case-control study*, Taiwan, 1999-2013
Table 3.
Association between antidepressant exposure and IFN treatment in a population-based case-control study*, Taiwan, 1999-2013
| Antidepressant exposure | Case (%) | Control (%) |
Unadjusted analysis |
Adjusted analysis |
||
|---|---|---|---|---|---|---|
| Odds-ratio (95% CI) | p | Odds-ratio (95% CI) | p | |||
| cDays | <0.0001† | <0.0001† | ||||
| 0 | 11,932 (77.01) | 58,143 (75.05) | 1.00 | 1.00 | ||
| 1-90 | 2,241 (14.46) | 11,908 (15.37) | 0.92 (0.87-0.96) | 0.92 (0.87-0.96) | ||
| 91-365 | 713 (4.60) | 3,713 (4.79) | 0.91 (0.87-0.96) | 0.93 (0.85-1.01) | ||
| >365 | 609 (3.93) | 3,711 (4.79) | 0.80 (0.73-0.87) | 0.79 (0.72-0.86) | ||
| cDDD | <0.0001† | <0.0001† | ||||
| 0 | 11,932 (77.01) | 58,143 (75.05) | 1.00 | 1.00 | ||
| 1-90 | 2,712 (17.50) | 14,333 (18.50) | 0.92 (0.88-0.96) | 0.92 (0.88-0.96) | ||
| 91-365 | 476 (3.07) | 2,646 (3.42) | 0.92 (0.88-0.96) | 0.87 (0.78-0.96) | ||
| >365 | 375 (2.42) | 2,353 (3.04) | 0.77 (0.69-0.86) | 0.77 (0.69-0.86) | ||
REFERENCES
2. Shiffman ML, Morishima C, Dienstag JL, Lindsay KL, Hoefs JC, Lee WM, et al. Effect of HCV RNA suppression during peginterferon alfa2a maintenance therapy on clinical outcomes in the HALT-C trial. Gastroenterology 2009;137:1986-1994.



3. NIH Consensus Statement on Management of Hepatitis C. 2002. NIH Consens State Sci Statements. 2002;19:1-46.

4. Okanoue T, Minami M, Makiyama A, Sumida Y, Yasui K, Itoh Y. Natural course of asymptomatic hepatitis C virus-infected patients and hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol 2005;3:S89-S91.


5. Chu CJ, Lee SD. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol 2008;23:512-520.


6. Chen DS, Kuo GC, Sung JL, Lai MY, Sheu JC, Chen PJ, et al. Hepatitis C virus infection in an area hyperendemic for hepatitis B and chronic liver disease: the Taiwan experience. J Infect Dis 1990;162:817-822.



7. Lee SD, Chan CY, Wang YJ, Wu JC, Lai KH, Tsai YT, et al. Seroepidemiology of hepatitis C virus infection in Taiwan. Hepatology 1991;13:830-833.


8. Chuang WL, Chang WY, Lu SN, Lin ZY, Chen SC, Hsieh MY, et al. The role of hepatitis C virus in chronic hepatitis B virus infection. Gastroenterol Jpn 1993;28(Suppl 5):23-27.



9. Lu SN, Chue PY, Chen HC, Wu MH, Chen IL, Huang JF, et al. Different viral aetiology of hepatocellular carcinoma between two hepatitis B and C endemic townships in Taiwan. J Gastroenterol Hepatol 1997;12:547-550.


10. Wang JH, Lu SN, Wu JC, Huang JF, Yu ML, Chen SC, et al. A hyperendemic community of hepatitis B virus and hepatitis C virus infection in Taiwan. Trans R Soc Trop Med Hyg 1999;93:253-254.



11. Adinolfi LE, Nevola R, Lus G, Restivo L, Guerrera B, Romano C, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol 2015;21:2269-2280.



12. Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-355.


13. Falck-Ytter Y, Kale H, Mullen KD, Sarbah SA, Sorescu L, McCullough AJ. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med 2002;136:288-292.


14. Restrepo A, Johnson TC, Widjaja D, Yarmus L, Meyer K, Clain DJ, et al. The rate of treatment of chronic hepatitis C in patients co-infected with HIV in an urban medical centre. J Viral Hepat 2005;12:86-90.


15. Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis 2003;36:97-100.



16. Evon DM, Esserman DA, Bonner JE, Rao T, Fried MW, Golin CE. Adherence to PEG/ribavirin treatment for chronic hepatitis C: prevalence, patterns, and predictors of missed doses and nonpersistence. J Viral Hepat 2013;20:536-549.



17. Evon DM, Simpson KM, Esserman D, Verma A, Smith S, Fried MW. Barriers to accessing care in patients with chronic hepatitis C: the impact of depression. Aliment Pharmacol Ther 2010;32:1163-1173.



18. Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut 2007;56:385-389.


19. Schaefer M, Capuron L, Friebe A, Diez-Quevedo C, Robaeys G, Neri S, et al. Hepatitis C infection, antiviral treatment and mental health: a European expert consensus statement. J Hepatol 2012;57:1379-1390.


20. Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry 2000;157:867-876.


21. Yan KK, Wong GL, Wong VW, Chan HL. Rate and factors affecting treatment uptake of patients with chronic hepatitis C in a tertiary referral hospital. Dig Dis Sci 2010;55:3541-3547.



22. Mendes-Correa MC, Martins LG, Ferreira PA, Tenore S, Leite OH, Leite AG, et al. Barriers to treatment of hepatitis C in HIV/HCV coinfected adults in Brazil. Braz J Infect Dis 2010;14:237-241.


23. Chou PH, Lin CC, Lin CH, Loh el-W, Chan CH, Lan TH. Prevalence of allergic rhinitis in patients with attention-deficit/hyperactivity disorder: a population-based study. Eur Child Adolesc Psychiatry 2013;22:301-307.



24. Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013;31:1514-1521.


25. Yang YH, Chen WC, Tsan YT, Chen MJ, Shih WT, Tsai YH, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol 2015;63:1111-1117.


26. Wacholder S, Silverman DT, McLaughlin JK, Mandel JS. Selection of controls in case-control studies. III. Design options. Am J Epidemiol 1992;135:1042-1050.



27. WHO. World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC classification index with DDDs. Available at: http://www.whocc.no/atc_ddd_publications/atc_ddd_index/. Accessed May 20, 2013.
28. Sockalingam S, Sheehan K, Feld JJ, Shah H. Psychiatric care during hepatitis C treatment: the changing role of psychiatrists in the era of direct-acting antivirals. Am J Psychiatry 2015;172:512-516.


29. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One 2014;9:e101554



30. Kramer JR, Kanwal F, Richardson P, Giordano TP, Petersen LA, El-Serag HB. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol 2011;106:483-491.



31. Chainuvati S, Khalid SK, Kancir S, Shea M, Edwards J, Sernyak M, et al. Comparison of hepatitis C treatment patterns in patients with and without psychiatric and/or substance use disorders. J Viral Hepat 2006;13:235-241.


32. Schaefer M, Sarkar R, Diez-Quevedo C. Management of mental health problems prior to and during treatment of hepatitis C virus infection in patients with drug addiction. Clin Infect Dis 2013;57 Suppl 2:S111-S117.



33. Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis 2013;207(Suppl 1):S19-S25.




34. Kanwal F, Hoang T, Spiegel BM, Eisen S, Dominitz JA, Gifford A, et al. Predictors of treatment in patients with chronic hepatitis C infection - role of patient versus nonpatient factors. Hepatology 2007;46:1741-1749.


35. Nelligan JA, Loftis JM, Matthews AM, Zucker BL, Linke AM, Hauser P. Depression comorbidity and antidepressant use in veterans with chronic hepatitis C: results from a retrospective chart review. J Clin Psychiatry 2008;69:810-816.