Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature
Article information
Abstract
Objective
Physical or mental imbalance caused by harmful stimuli can induce stress to maintain homeostasis. During chronic stress, the sympathetic nervous system is hyperactivated, causing physical, psychological, and behavioral abnormalities. At present, there is no accepted standard for stress evaluation. This review aimed to survey studies providing a rationale for selecting heart rate variability (HRV) as a psychological stress indicator.
Methods
Term searches in the Web of Science®, National Library of Medicine (PubMed), and Google Scholar databases yielded 37 publications meeting our criteria. The inclusion criteria were involvement of human participants, HRV as an objective psychological stress measure, and measured HRV reactivity.
Results
In most studies, HRV variables changed in response to stress induced by various methods. The most frequently reported factor associated with variation in HRV variables was low parasympathetic activity, which is characterized by a decrease in the high-frequency band and an increase in the low-frequency band. Neuroimaging studies suggested that HRV may be linked to cortical regions (e.g., the ventromedial prefrontal cortex) that are involved in stressful situation appraisal.
Conclusion
In conclusion, the current neurobiological evidence suggests that HRV is impacted by stress and supports its use for the objective assessment of psychological health and stress.
INTRODUCTION
Hans Selye defined stress as “a response to change in order to maintain the state of stability or homology that the body has maintained against the stimulus to break the mental and physical balance and stability of the body.” [1] Stress was also defined by Kenneth Hambly as a maladaptive state in which the sympathetic nervous system is overactivated, causing acute or chronic physical, psychological, and behavioral impairment [2]. The search for stress biomarkers remains a challenging task for researchers and clinicians as there are several obstacles. One obstacle is a lack of consensus on the definition of stress. Moreover, we lack a comprehensive framework for investigating how organisms function in and adapt to constantly changing environments [3]. At present, there is no universally recognized standard for stress evaluation. A number of studies using existing stress measurement methods (e.g., psychological measures of stress) and examining biological markers (e.g., cortisol, amylase) have been performed. Moreover, studies on heart rate variability (HRV) and stress are increasing in frequency. HRV is the fluctuation of the length of heart beat intervals [4]. HRV represents the ability of the heart to respond to a variety of physiological and environmental stimuli [5]. Low HRV conveys a monotonously regular heart rate. Moreover, low HRV is associated with impaired regulatory and homeostatic autonomic nervous system (ANS) functions, which reduce the body’s ability to cope with internal and external stressors. Thus, HRV is a noninvasive electrocardiographic method that can be used to measure the ANS in a variety of clinical situations (e.g., during psychological stress evaluations) [6]. Many researchers have conducted studies that used HRV to measure stress, operating under the assumption that HRV is a reliable index of stress. However, few studies have confirmed whether HRV is a good indicator of stress. In this review, we examined the literature providing a rationale for selecting HRV as a reliable indicator of psychological stress. The value of HRV as a stress indicator must be assessed to support its future clinical use as a noninvasive and simple diagnostic test.
Methods
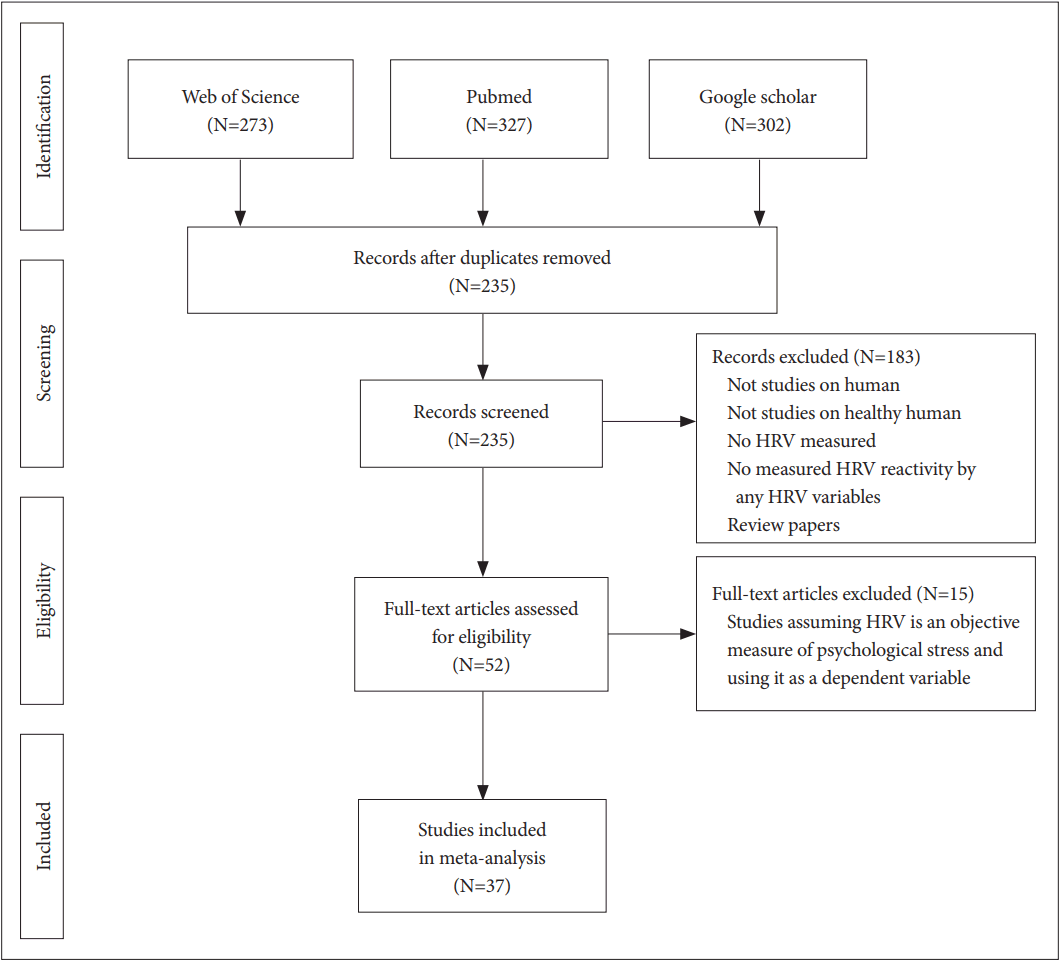
The Web of Science® (WoS), National Library of Medicine (PubMed), and Google Scholar databases were searched for articles published from 2007 to 2017 pertaining to psychosocial stress and HRV. The following combination of terms was used for each database search: 1) WOS, “[Title (TI)=(stress) AND TI=(‘Heart rate’ OR ‘Heart Rate Variability’ OR ‘HRV’ OR ‘Cardiac Vagal Control’ OR ‘Cardiac Vagal Tone’ OR ‘Autonomic Nervous System’ OR ‘Cardio-Vascular Reactivity’)]”; 2) PubMed, “stress (Title) AND [‘HRV’ (Title/Abstract) OR ‘Heart Rate Variability’ (Title/Abstract)]”; and 3) Google Scholar, “allintitle: stress AND (‘Heart Rate Variability’ OR ‘HRV’).” The inclusion criteria were that each study involving human participants, used HRV as an objective measure of psychological stress, and measured HRV reactivity by evaluating any HRV variables calculated using frequency-based or time-based measures. Secondary references and other literature providing theoretical evidence for selecting HRV as a stress indicator or the role of the ANS in psychological stress and heart rate were also included in the study. Each of the database searches yielded 107 studies matching the inclusion criteria. Subsequently, we excluded studies that did not concur with the purpose of the literature review (e.g., studies assuming HRV is an objective measure of psychological stress and using it as a dependent variable). Ultimately, 37 studies meeting our criteria were selected for this review (Figure 1).
Results
Autonomic regulation of psychological stress conditions
The two main pathways by which psychological stress affects the body are the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) [7]. The ANS and HPA axis are highly coordinated and interconnected [8]. The ANS quickly promotes physiological changes through the SNS and parasympathetic nervous system (PNS). The PNS promotes the sympathetic response to stress, commonly referred to as a fight or flight response, by withdrawing the inhibitory effect [9]. A series of changes follows, including the release of noradrenaline from the locus coeruleus [10]. During the stress response, the HPA axis triggers a series of endocrine changes, beginning with the release of corticotropin releasing hormone from the hypothalamus [8,11]. Specifically, the PNS plays an important role in alleviating the stress response of individuals by inhibiting or inhibiting the SNS and HPA axis [8]. Stress is associated with variations in autonomic activity that disrupt homeostatic processes [9]. The ANS responds to the needs of the internal viscera as well as external stimuli. Homeostasis is associated with the regulation of internal viscera, whereas the stress response prioritizes external stimuli over internal needs. Thus, stress occurs when an organism’s physiological demands are no longer adequately fulfilled by the PNS. Consequently, the measurement of parasympathetic tone may serve as an index of stress and stress vulnerability. Moreover, stasis, which is a lack of endogenous variability in neurally mediated peripheral systems (e.g., heart rate), is a sign of severe physiological distress [9].
The homeostatic functions of the ANS are demonstrated by the baroreceptor-heart rate reflex, a reciprocal change in the activities of the ANS [12]. Baroreceptor reflexes are organized largely within lower central autonomic substrates at the brainstem level. However, through the evolutionary development of the rostral brain systems, these lower autonomic systems became integrated with higher neural networks [13]. The limbic system and forebrain including the hypothalamus, amygdala, and medial prefrontal cortex, have been shown to issue synaptic projections to brainstem reflex networks as well as to autonomic regions [12]. Stressors can lead to an inhibition or shift in the set point of baroreceptor reflexes [12,14]. Brainstem baroreceptor reflexes exert reciprocal control over the two autonomic branches (i.e., SNS and PNS) [12].
The reciprocal mode of autonomic control can vary under different circumstances. Using selective pharmacological blockades targeting the SNS and PNS, Berntson et al. [13] found that orthostatic challenges and standard psychological stressors produced distinct patterns of control. Responses to orthostatic stress showed minimal individual variation. In contrast, psychological stressors produced widespread individual differences in the autonomic response. In response to psychological stressors, participants either showed sympathetic activation, vagal withdrawal, or a reciprocal pattern of autonomic response [13]. These findings show that individuals differ in how they respond to psychological stressors.
Autonomic cardiac control
Although cardiac automaticity is intrinsic to various pacemaker tissues, heart rate (HR) and rhythm are largely under the control of the ANS [15]. HR is controlled by the balancing action of the SNS and PNS branches. The parasympathetic influence on HR is mediated by the release of acetylcholine from the vagus nerve. Muscarinic acetylcholine receptors respond to this release by increasing cell membrane K+ conductance [16,17]. The sympathetic influence on HR is mediated by the release of epinephrine and norepinephrine. β-adrenergic receptors are activated by the release of these hormones, which results in cAMP-mediated phosphorylation of membrane proteins [18]. Increased SNS or diminished PNS activity results in cardioacceleration, whereas decreased SNS or increased PNS activity causes cardio-deceleration [5]. The SNS mainly acts on the ventricular muscles and increases their contractility. Moreover, the SNS increases the excitation frequency, excitation conduction velocity, and excitability of the sinoatrial (SA) node. When the SNS is maximally stimulated, the magnitude of the HR and contractility can triple and double, respectively. The PNS primarily acts on the SA and atrioventricular (AV) nodes to reduce HR. Vagal and sympathetic activity constantly interacts. As the SA node is rich in acetylcholinesterase, the effect of the vagal impulse is brief, owing to rapid hydrolysis of acetylcholine [19,20]. Under resting conditions, vagal tone prevails over sympathetic activity, and variations in the heart period are largely dependent on vagal modulation. When the SNS and PNS are removed, the HR rises above steady-state rates. Vagal dominance occurs when the vagus nerve, which is a parasympathetic nerve in the stable state, is more active than sympathetic nerves. Dysregulation of the autonomic nervous control of the cardiovascular system is associated with increased sympathetic and reduced parasympathetic tone and plays an important role in coronary artery disease and the genesis of potentially lethal ventricular arrhythmias [6,21]. Separate rhythmic contributions from sympathetic and parasympathetic autonomic activity modulate the HR intervals of the QRS complex in the electrocardiogram (ECG) at distinct frequencies. In this context, the degree of variability in the HR provides information about the functioning of nervous control on the HR and the heart’s ability to respond. Since the heart is not a metronome and its beats are irregular, HRV is normal and to be expected. Moreover, HRV indicates the heart’s ability to respond to multiple physiological and environmental events, (e.g., breathing, physical exercise, mental stress, hemodynamic and metabolic changes, and sleep and orthostatism) and compensate for disease-induced disorders [5,22-25]. HRV can be used as a valuable tool to measure the sympathetic and parasympathetic function of the ANS [5].
Theoretical significance of heart rate variability
Long before the modern technology of HRV was invented, physicians recognized the potential importance of heart rhythms. In 1847, Ludwig was able to observe a quickening of pulse rate with inhalation and a slowing with exhalation in the dog [26]. This was the first report of respiratory sinus arrhythmia (RSA). Moreover, in 1868, Donders examined the relationship between respiration, HR, and the vagus nerve [27]. In 1920, Bainbridge explained RSA in terms of alterations in baroreceptor and volume receptor responses associated with respiratory alterations in thoracic pressure [28]. Moreover, in 1915, the studies of Eppinger and Hess focused on clinical issues related to putative abnormalities in autonomic functions. They focused on the potential role of the ANS in atypical physiological responses and clinical disorders. Their studies also emphasized the activity of the vagus nerve, which may allow pharmacological manipulations and potential treatments [29]. In 1967, Wolf viewed HRV as reflecting brain-vagal-heart communication [30]. Wolf provided an important connection between clinical research and psychophysiology. After that, HRV was treated as a descriptive variable without being attributed to any specific physiological state. At present, understanding of HRV interpretation is increasing, and we are also aware of the interaction between HRV’s underlying physiological mechanisms and behavioral processes [29].
HRV variables are summarized in Tables 1 and 2 [15]. In 1996, the Task Force of the European Society of Cardiology (ESC) and the North American Society of Pacing and Electrophysiology (NASPE) defined and established standards for the measurement, physiological interpretation, and clinical use of HRV. Time-domain and frequency-domain indices and geometric measures are standard clinical parameters [15]. Time-domain analysis measures variation in HR over time or the intervals between successive normal cardiac cycles. Time-domain analysis of recording data involves simple calculations of mean normal-to-normal (NN) intervals and the variance between NN intervals. One of the simplest time-domain analysis variables is the standard deviation of the NN interval (SDNN; i.e., the standard deviation of NN). When HRV is large and irregular, the SDNN value increases. Therefore, SDNN is an index of physiological resilience against stress. In contrast to SDNN, which is computed directly from the NN interval, the root mean square of the successive differences (RMSSD), number of interval differences of successive NN intervals greater than 50 ms (NN50), and proportion derived by dividing NN50 by the total number of NN intervals (pNN50) are derived from the difference between adjacent NN intervals. These variables are impacted by the PNS, as they reflect beat-to-beat changes [31,32]. Power Spectral Density (PSD) analysis of the frequency domain provides information about how power is distributed (i.e., the variance) as a function of frequency, which allows autonomic balance to be quantified at any given time. By using PSD analysis to understand HRV, we can distinguish between the activity of the SNS and PNS. Frequency-domain analysis is preferred for short-term measurements (i.e., 5 min). PSD analysis allows the intensity of the HRV spectral components [i.e., the high-frequency band (HF), low-frequency band (LF), and very low frequency band (VLF)] to be determined. Different HRV spectral components are associated with either the sympathetic or parasympathetic branches of the ANS. The HF is a measure of PNS activity, as it reflects the activity of the vagus nerve, whereas LF reflects the activity of the SNS [33,34].
Studies of HRV reactivity to psychological stressors in healthy human participants
Sloan et al. [35] analyzed 24-h electrocardiographic recordings from 33 healthy participants to examine the association between the RR interval, HRV responses, and periodic diary entries measuring physical position, negative affect, and time of day. As expected, their results showed that increases in stress were associated with decreases in the RR interval. Moreover, psychological stress was significantly associated with an increase in the LF/HF ratio, suggesting increased SNS activity during stressful periods of the day [35]. Using a questionnaire survey and short-term HRV recordings of 223 healthy male white-collar workers, Kageyama et al. [36] investigated the relationship between the number of job stressors, self-reported sleep quality, and daytime autonomic activities. They found no correlation between the HRV parameters and five job stressor scores [36]. However, subsequent studies have reported that some HRV indicators reflect psychological stress. Studies of healthy human individuals that examined HRV variation associated with psychologically stressful situations are summarized in Table 3. There was heterogeneity among the studies concerning the type of stress-eliciting task used and HRV reactivity (i.e., laboratory environment task or subjective stress reports). Moreover, the HRV reactivity studies were either short-term (5 min) or continuous 24-h studies. In most studies, HRV variables changed in response to stress induced by various methods. However, the presence of significant variation in HRV variables was inconsistent. The most frequently reported factor associated with variation in HRV variables was low parasympathetic activity, which is characterized by a decrease in the HF and an increase in the LF. Dimitriev et al. [37] recently reported that mental stress leads to an increase in predictability, RR interval regularity, and reduced complexity. This reflects a change toward more stable and periodic HR behavior under stress. Reduced HRV and inhibited parasympathetic activation increase vulnerability to future stress.
Neurobiological evidence of HRV as a stress measurement
Many of the studies examined were consistent with Claude Bernarde’s (1865) finding that the vagus nerve serves as a structural and functional link between the brain and the heart. His work was among the earliest to systematically investigate the connections between the brain and the heart [38]. Perceptions of threat and safety are common core elements of “stressors.” Continuous perception of threats is harmful to the human body and affects the regulation of hippocampal circuits, endocrine systems, ANS, and others [39-42]. If an organism is to avoid a chronic state of threat, it is essential to determine whether threat assessment is appropriate depending on the context. The prefrontal cortex (PFC) and medial PFC (mPFC), in particular, appear to be important in this appraisal process [3,38]. In safe contexts, threat representations in the amygdala would be inhibited by the ventromedial PFC (vmPFC). A manipulation of the vmPFC, such as pharmacological intervention, can help to inhibit subcortical threat circuits and reduce stress responses [43-46]. Studies have shown reciprocal inhibition of the PFC and amygdala [47-49]. The aim of this study was to investigate the relationship between HRV and neurogenic rhythms. These inhibitory prefrontal processes can be assessed using measures of vagal function, such as HRV. A meta-analysis of HRV neuroimaging studies found a link between HRV and brain regions (e.g., the ventral aspect of the mPFC) associated with reduced threat perception [3]. HRV can measure the degree of functional integration of the axes connecting vmPFC, brainstem and peripheral anatomy and can represent the degree to which it provides flexible control over ANS.
Clinical applications of HRV
In view of observations of stress-associated variation in HRV and existing neurobiological evidence, HRV may be used as an objective assessment of stress and mental health. However, since psychiatric illnesses have numerous causes and symptoms, consistent biological measurements are difficult to acquire in individuals with mental illness. Thus, a patient’s psychological and medical history should be equally considered when interpreting HRV results. Therefore, HRV can be considered a tool that reflects heart activity and overall autonomic health, rather than specific mental illnesses or disease states. Since the concept of stress includes biological and psychological factors, objective and physiological evaluations as well as self-reporting should be integrated when evaluating stress, using HRV in clinical practice. Many studies have found an association between mental health and HRV. However, since HRV is associated with various stress factors, stress duration, individual coping ability, and lifestyle habits, these studies are difficult to interpret. Many physical conditions and lifestyle habits can affect HRV results, including physiological factors (e.g., breathing, circadian rhythms, and posture), non-modifiable factors (e.g., age, sex, and genetic factors), modifiable lifestyle factors (e.g., obesity, metabolic syndrome, physical activity, smoking, and drinking), and other factors [e.g., medication (e.g., anticholinergics, stimulants, and beta-blockers)] [50-55].
Hans Seyle [1,56] proposed a three-stage stress response model. The first stage is the “alarm reaction stage,” in which the body reacts to a stressor with the fight-or-flight response and activates the SNS. The second stage is the “resistance stage,” in which the body adapts to the stressor. During this stage, the PNS restores many physiological functions to normal, while the body focuses its resources against the stressor. Although the outward appearance of the organism seems normal, blood glucose, cortisol, and adrenalin levels remain elevated. If a stressor continues beyond the body’s capacity to cope, the organism exhausts its resources, making it susceptible to disease or death. This “exhaustion stage” is reached when the acquired adaptation or resistance is lost. When assessing the severity of a patient’s stress level in a clinical setting, HRV results should be interpreted with this three-stage process in mind. At each stage, stress causes changes in physiological function, which are reflected in HRV changes. Due to the variety of potential stressors and individual stress responses, it is essential to understand the overall autonomic context and examine a patient’s medical and psychological history when interpreting the relationship between HRV and stress.
Discussion
HRV is sensitive to changes in ANS activity (i.e., changes in the SNS and PNS) associated with stress. In most studies, HRV variables changed in response to stress induced by various methods. The most frequently reported factor associated with variation in HRV variables was low parasympathetic activity, which is characterized by a decrease in the HF and an increase in the LF. HRV may be associated with the activity of a flexible network of neural structures, which are dynamically organized in response to environmental challenges. Indeed, neuroimaging studies suggest that HRV may be linked to reduced threat perception, mediated by cortical regions (e.g., the ventral aspect of the mPFC) involved in the appraisal of stressful situations. In clinical situations, HRV can be considered a tool that reflects heart activity and overall autonomic health, rather than specific mental illnesses or disease states. Thus, when evaluating the relationship between stress and HRV, it is essential to consider the overall autonomic context as well as the patient’s medical and psychological history.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP: Ministry of Science, ICT, and Future Planning) (No. 216C000671).