Comparative Analysis of the WISC between Two ADHD Subgroups
Article information
Abstract
Objective
The prevalence of attention deficit/hyperactivity disorder (ADHD) in school-age children is 7.2%, and ADHD is divided into clinical subtypes.
Methods
The current study explored whether specific cognitive profiles as assessed using the Wechsler Intelligence Scale for Children (WISC)-IV could be obtained for each clinical ADHD subtype (ADHD-Inattentive type and ADHD-Combined type) and investigated the correlation between WISC scores and parental age at their children’s birth or birthweight. The enrolled sample comprised 12 ADHD-I and 15 ADHD-C subjects.
Results
An impaired Processing Speed Index was found in ADHD-I. The age of the father at the child’s birth and birthweight positively correlated with the full scale intelligence quotient (FSIQ) score in the WISC assessment.
Conclusion
Inattentiveness within the behaviors of the children with ADHD-I is partly due to the impaired processing speed, therefore effective support for ADHD will be conducted if educator decreases their speaking speed. Since biological basis of ADHD is still largely unknown, future studies using both psychological and biological methods will reveal the etiology of ADHD. These scientific assessments will provide information for more effective approaches in the care of children with ADHD.
INTRODUCTION
Attention deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder in school-age children, with a prevalence of 7.2% [1]. However, symptom presentation is not limited to school ages. Based on a national survey in the US [2], 4.4% of the adult population satisfies the DSM-IV ADHD diagnostic criterion. However, the risk of overdiagnosing ADHD or excessive medication has recently become a substantial issue [3]. Historically, the formulation of the concept of ADHD began with a case report by Sir GF Still in 1902 [4]. Still reported 43 cases with “defect of moral control associated with general impairment of intellect,” which represents the hyperactive or impulsive behavioral feature of ADHD in childhood. In 1980, attention deficit disorder (ADD) was included in the DSM-III. ADD subtypes (ADD/H with hyperactivity or ADD/WO without hyperactivity) should be added to the criteria for ADD diagnosis. In the revised text of the DSM-III (DSM-IIIR), the first description of ADHD was added, and the recently updated criterion, the DSM-5, has inherited the concept of ADHD. Due to the historical process, the subtypes, such as ADHD-H (primarily hyperactive/impulsive), ADHD-I (primarily inattentive), or ADHD-C (combined), should be identified at ADHD diagnosis. For example, several studies have found more severe and internalized symptoms in children with ADHD-C than those in children with ADHD-I [5,6] because children with ADHD-I have only impaired attention, whereas children with ADHD-C have impairments in both hyperactivity-impulsivity and attention [7]. Additionally, practical care is different among the subtypes. As detecting ADHD subtypes is crucial for planning medication, prognostic prediction and improving quality of life, ADHD must be evaluated not only on the basis of symptoms but also with psychological assessments, including cognitive batteries.
In the current retrospective survey, we aimed to identify different features between two ADHD subtypes (ADHD-C and ADHD-I) using the Wechsler Intelligence Scale for Children (WISC) assessment. The WISC is typically administered to children (aged 5 years and older) and adolescents (less than 17 seventeen years old) to evaluate their full scale intelligence quotient (FSIQ), which represents children’s general intellectual ability and cognitive functions. The WISC assessment consists of four factors: the Processing Speed Index (PSI), the Verbal Comprehension Index (VCI), the Perceptual Reasoning Index (PRI), and the Working Memory Index (WMI) (Figure 1).
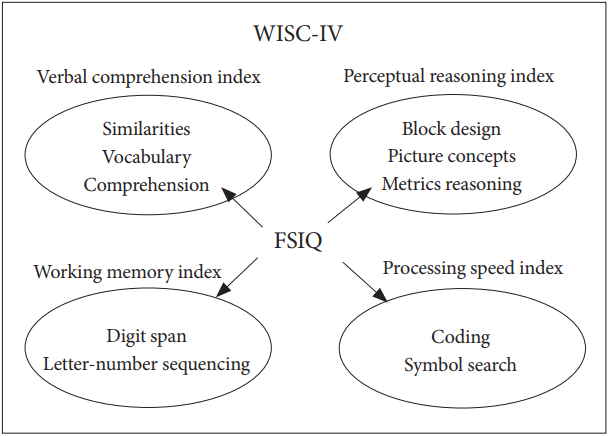
The components of the four WISC-IV subscales. WISC: Wechsler Intelligence Scale for Children, FSIQ: full scale intelligence quotient.
Another aim was to reveal the relationship between parental age at the children’s birth and the subtypes or the subscale scores on the WISC. Recent reports have suggested a higher incidence rate of ADHD (13.1-fold) if the father is more than 45 years old at the child’s birth [8]. Similarly, compared with fathers aged 25–29 years old, fathers less than 20 years old have an increased risk of having children with ADHD (1.5-fold) [9]. These inconsistent reports motivated us to determine the association between parental age at ADHD children’s birth and cognitive features in ADHD. In addition, we evaluated the relationship between birthweight and WISC scores, including the FSIQ and four subclassifications.
METHODS
During 2014, children meeting the DSM-IV ADHD criteria who visited Han-nan Hospital were analyzed. Han-nan Hospital is located in the west of Japan and has a pediatric psychiatric unit (maximum capacity: 25 children). Each day, an average of 15.1 outpatients visit the pediatric psychiatry hospital, and 1.1 children become new patients.
ADHD patients with a FSIQ (assessed by WISC) less than 70 were omitted from further analysis. Twenty-seven patients (22 boys and 5 girls; average age of 9.7 years) were selected for clinical record collection (Table 1). This retrospective analysis was approved by the Ethical Committee at Han-nan Hospital (H27-04).
Statistical procedures included correlation analysis of the relationship between WISC results and parental age or birthweight. Unpaired t-test was used when the two assigned groups (ADHD-I and ADHD-C) were compared in terms of the results from the WISC subscales. Data were analyzed using JMP Pro® statistical software.
RESULTS
Twenty-seven patients were assigned as ADHD-I (n=12) or ADHD-C (n=15) based on the DSM-IV criterion. The average parental age at birth was 31.2 years for fathers and 28.6 years for mothers (n=26, respectively). Unpaired t-tests revealed significant differences between the two groups (ADHD-I and ADHD-C) on the PSI (t=-0.25, p<0.05) but not FSIQ. On the subscale analysis, a significant difference was found only for the Coding scale subtype (t=-2.2, p<0.05) (Table 2).
The correlation analysis comparing parental age at birth and birthweight with the FSIQ and WISC revealed that FSIQ and father’s age at birth were significantly correlated (r=0.50, p=0.0091) (Figure 2). The subscale analysis indicated that both the PRI (r=0.46, p=0.0174) and WMI (r=0.49, p=0.0116) of the WISC correlated with father’s age at the child’s birth. In addition, the birthweight of the 27 patients was significantly correlated with the FSIQ (r=0.40, p=0.0364) (Figure 3) and WMI (p=0.0149) within the subscales. Moreover, if birthweight was analyzed only, including full-term births (n=20, birth between 37 weeks and 41 weeks), the FSIQ, PRI (p<0.01), VCI, WMI, and PSI (p<0.05) were correlated (Table 3).
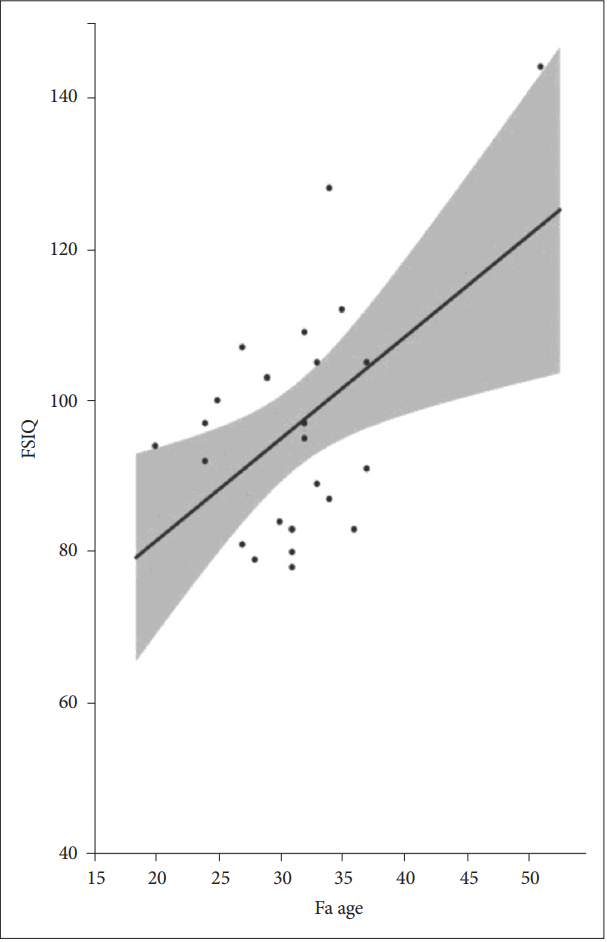
Scatterplot of FSIQ and father’s age at ADHD child’s birth (N=27). FSIQ: full scale intelligence quotient, ADHD: attention deficit/hyperactive disorder.

Scatterplot of FSIQ and bodyweight at ADHD child’s birth (N=27). FSIQ: full scale intelligence quotient, ADHD: attention deficit/hyperactive disorder.
DISCUSSION
The purpose of the current retrospective survey was to evaluate WISC scores among children with ADHD. Determining levels of intelligence in children with ADHD-I and ADHD-C will provide information for more appropriate involvement in their care. Treatment strategies are slightly different among ADHD subtypes. For instance, moderate to high doses of methylphenidate (Ritalin) are used for children with ADHD-C, whereas low doses are helpful for most children with ADHD-I [10].
The results indicate similar FSIQ scores between the two subtypes on the WISC, whereas PSI scores were significantly higher in ADHD-C than those in ADHD-I. Processing speed represents how quickly a person can perform simple or automatic cognitive tasks; thus, children with hyperactive behavior showed faster speeds on the cognitive tasks. The higher PSI scores in ADHD-C are consistent with a previous study [11]. Thaler et al. [12] reported that WISC profiles in children with ADHD fit five cluster models in hierarchical cluster analysis. Within the clusters, two clusters (named Reduced PSI and Below Average WMI/PSI) indicated that low WISC-IV PSI was associated with ADHD-I. In other words, children with a lower PSI were recognized as inattentive. A similar trend in the PRI was found, although the difference was not significant. A higher PRI in ADHD-C indicates better outcomes with an educational approach that includes visualization or modeling, especially for children with ADHD-C. By contrast, the VCI is the only subscale in which the scores of ADHD-I exceed those of ADHD-C. This finding potentially suggests that ADHD-I affects verbal and auditory communication.
ADHD-associated inattentiveness is believed to be due to a deficit in the locus coeruleus-norepinephrine system (LC-NE system [13]) because differences in P3 (synonymous name: P300, Event Related Potential) amplitudes has been frequently found in people with inattentiveness [14-16]. However, concluding that inattentiveness in ADHD is directly related to altered P3 amplitudes is difficult because deficits in P3 amplitudes are observed not only in ADHD-I patients but also in boys with disinhibitory disorders, such as conduct disorder, antisocial personality disorder, alcoholism, or nicotine dependence [17]. The locus coeruleus (ceruleus) is located in the pons and is involved in physiological responses to stress or damage. Through the release of norepinephrine, the LC-NE system regulates many brain functions involved in attention, memory, emotions, and behavioral flexibility [18,19]. Similar findings have also been obtained with electroencephalogram (EEG) measurements. Previous research has shown that alpha wave patterns in individuals with ADHD are different from those in age-matched controls [20]. Furthermore, increased theta and slower alpha waves, which are associated with inefficient brain processing or disturbed awareness, have been repeatedly found in subjects with ADHD; however, this feature is not unique to ADHD [21]. People with obsessive compulsive disorders, learning disorders, or some types of dementia tend to show similar EEG patterns. Inattention is one of the domains regulated by the LC-NE system; therefore, the specific etiology of inattentiveness in ADHD patients should be studied more intensively with an electrophysiological approach.
Additional studies using functional neuroimaging approaches, such as SPECT, PET and fMRI, have repeatedly detected associations between ADHD and abnormalities in the fronto-striatal circuitry, including the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), dorsal anterior cingulate cortex (dACC), and striatum [21]. Although the apparent deficit in fronto-striatal circuitry may be downstream of the initially impaired brain regions, ADHD pathology is at least related to this circuitry. Beyond the fronto-striatal model, newer approaches with Diffusion Tensor Image (DTI) of the default mode network have shown deficits in many other brain regions, such as visual networks. For instance, a 33-year follow-up study of ADHD children detected decreased cortical thickness in the medial occipital cortex [22]. Although abnormalities in the medial occipital cortex (BA18, BA19) have been repeatedly shown in earlier neuroimaging studies [23,24], sufficient attention has not been given to this region. High-resolution MRI has indicated reduced gray matter volume in the bilateral occipital cortex [25] in people with ADHD (n=31) compared with healthy controls (n=31); therefore, the reduced PSI could be partly explained by impaired visual function in individuals with ADHD.
Inattentiveness in ADHD-I is characterized by a “sluggish cognitive tempo” (SCT) [26-28]. SCT items on the DSM-IV Field Trial for ADHD were discarded due to insufficient predictive power to distinguish ADHD subtypes [29]; however, SCT has been re-evaluated because SCT is consistent with the features of sluggishness, drowsiness, spaciness, lethargy, and hypoactivity in children with ADHD-I [10]. Additionally, SCT refers to frequently observed conditions such as daydreaming, having difficulty staying awake in certain situations, and becoming easily bored, easily confused or more lethargic than others. A recent systematic review of SCT [30] has reported that SCT affects an individual’s cognitive functioning (e.g., social interactions or academic achievement), but the lack of standards in SCT measurements across studies was also revealed. Moreover, SCT has a hypothesized link to impaired processing speed. Most studies [5,26,31,32] have suggested a link between SCT and slow processing speed; however, some researchers have questioned whether neuropsychological/behavioral assessments of cognitive tempo have been applied [33]. The lowed processing speed in ADHD-I found in our study is based on WISC performance. Thus, further assessment using an appropriate SCT measurement is warranted in the future.
Regarding the parental ages at birth of the children with ADHD, self-reported father’s ages at the child’s birth and the FSIQ from the sample were significantly associated (r=0.50, p=0.0091), whereas no significant correlation with mother’s age was found. Recent epidemiological studies in Sweden (2.6 million people [8], and Denmark (3 million people) [9] have found higher incidence rates of schizophrenia and autism with older fathers (>45 years old). However, the incidence of ADHD is slightly different from the incidence of these two disorders. Offspring born to men over the age of 45 years were 5% and 43% less likely to have a diagnosis of ADHD in the Danish and Swedish studies, respectively [34]. As a reference group was not included in the current study, our findings cannot be treated as a replication of these two large-scale epidemiological studies. However, the FSIQ in the ADHD sample (n=27) was associated with the father’s age at the child’s birth. In general, an inverted U-shaped relationship is observed between paternal age and IQ of offspring [35]. A recent genomic analysis has found that de novo mutations carry a large effect on higher rates of autism spectrum disorder, which is regarded as one of the reasons why older paternal age increases the risk of schizophrenia or autism spectrum disorders in offspring. Future work including both genomic and psychological assessments of ADHD children will reveal the biological basis of the altered cognitive functions in ADHD in detail.
Several limitations should be addressed. Most importantly, a limited number of ADHD subjects were enrolled in this study. This limitation is due to the brief duration of the sample recruitment (1 year) of patients with first visits during 2014. Therefore, future studies should expand the recruitment duration to include more psychological analysis data. Another limitation is the lack of data from ADHD-H (primarily hyperactive/impulsive). Most previous studies have focused on the difference between ADHD-C and ADHD-I because cases with pure ADHD-H phenotypes are rarely seen in school-age children [1].
Children with ADHD-C adapt more easily to education with visualized methods (e.g., graphs, figures, and behavioral modeling); children with ADHD-I have a harder time adapting because of their limited PRI. Similar results will be obtained if educators choose verbal communication, although educators should decrease their speaking speed because we found worse PSI in children with ADHD-I on the current WISC assessment. A recent meta-analysis aimed at accurately estimating the prevalence of ADHD concluded that the prevalence is 7.2% and 6.6–7.8% at 95% CI [1]. ADHD prevalence varies among schools, cultures, and countries. Children with ADHD have trouble concentrating or sitting still in classrooms. Therefore, effective involvement in the care of children with ADHD is needed, and the current psychological assessment findings could be helpful for developing support for these children.


