Generalized Anxiety Disorder, Comorbid Major Depression and Heart Rate Variability: A Case-Control Study in Taiwan
Article information
Abstract
Objective
Decreased heart rate variability (HRV) has been reported in generalized anxiety disorder (GAD), but the results are mixed. Little is known about the impact of comorbid major depression (MD) on HRV in GAD patients. Both issues necessitate further investigation.
Methods
Twenty unmedicated, physically healthy GAD patients, 20 GAD patients with a secondary diagnosis of MD, 40 MD patients and 60 matched controls were recruited. We used the Hamilton Anxiety Rating Scale and the Hamilton Depression Rating Scale to assess anxiety and depression severity, respectively. Cardiac autonomic function was evaluated by measuring HRV parameters. Frequency-domain indices of HRV were obtained.
Results
Three patient groups had more anxiety and depression symptoms than control subjects, but heart rates (HRs) were significantly elevated only in GAD patients with comorbid depression. Relative to controls, GAD patients had reduced HRV while GAD patients with comorbid depression displayed the greatest reductions in HRV among three patients groups. Correlation analyses revealed anxiety/depression severity significantly associated with HRs, variance, LF-HRV and HF-HRV. However, separately analyzing among individual groups and adjusting for HRV-associated covariables rendered the correlations non-significant.
Conclusion
Our results suggest that reduction in HRV is a psychophysiological marker of GAD and individuals with comorbid GAD and MD may be distinguished based on psychophysiological correlates (for example, HF-HRV) from non-comorbid GAD patients. Taken into account that comorbid depression may confer increased risks for cardiovascular events in GAD patients, this subgroup of GAD patients may benefit better from cardiovascular risk reduction strategies.
INTRODUCTION
Generalized anxiety disorder (GAD) is a chronic anxiety disorder and characterized by fluctuating levels of persistent, uncontrollable worry associated with fatigue, insomnia, muscle tension, poor concentration, and irritability. Anxiety disorders have been associated with increased risks of cardiovascular morbidity.1 One potential explanation proposed for this association is a dysregulation of the autonomic control of the heart because autonomic nervous system (ANS) activity is associated with cardiovascular disease (CVD)2,3 and anxiety disorders.1 Many previous studies has examined the impact of phobic anxiety on patients with established coronary artery disease (CAD),1,4,5 few examine GAD-specific contribution. Until recently, there were two published GAD-specific studies quantified GAD as having a distinctly negative impact on outcome of patients with stable CAD.6,7 It raises the question of whether the relationship between GAD and the reported increase of cardiac risks is mediated by cardiac autonomic dysregulation. Because CVD itself might influence cardiac autonomic function, we focused on GAD patients without CVD, in order to avoid overestimation of the association between GAD and cardiac autonomic function.
The ANS links the central nervous system and the cardiovascular system. Efferent links in the neural control of heart rhythm consist of sympathetic and parasympathetic fibers innervating the sinus node. Because sympathetic and parasympathetic firing alters spontaneous sinus node depolarization, cardiac rate and rhythm convey information about autonomic influences to the heart.8 Previous research focused primarily on the sympathetic branch of the ANS and reported higher levels of sympathetic activity in GAD patients.9-13 However, few psychophysiologic studies of GAD use a technique enabling quantitative assessment of influences of the parasympathetic nervous system (PNS), the other branch the ANS. The PNS controls heart rate (HR) by brain stem regions that project to the heart via the vagus nerve. These vagal fibers functionally slow HR and actively inhibit the sympathetic influences to the heart.14 Vagal nerve activity thus leads to regular oscillations in HR. Cardiac vagal control (CVC), essentially, reflects the degree to which there is tonic vagal influence on the heart.15 Low CVC, which causes reduced autonomic flexibility, has been associated with a number of psychopathological states, including depression,15 anxiety,16 and the cognitive processes that have been associated with depression and anxiety, namely worrying.17 Given the central role of worry in the DSM-IV diagnostic criteria for GAD, CVC has recently become a promising candidate for investigation. Our study used frequency-domain analysis of heart rate variability (HRV), a method different from previous studies,9-13 providing indices that reflect sympathetic and parasympathetic cardiovascular influences.
Frequency-domain analysis of HRV, with its standard procedure and interpretation first reported in 1996, is a sophisticated and noninvasive tool for detection of ANS regulation of the heart.18 An important advantage of frequency-domain analysis of HRV is that it utilizes spontaneous fluctuations in HR to estimate tonic ANS functions. However, it should be noted that a well controlled condition is required for spontaneous ANS functional recordings. In the present study, we used spectral analysis of HRV while subjects were in supine rest in a quiet and relaxing atmosphere for 5-min short recording times. We did this because short-term resting-state measures of HRV better reflect intrinsic HRV.19
Studies have seldom examined the impact of GAD on HRV. As far as we know, the results of those studies concerning resting HRV in GAD are mixed. For example, some studies found GAD patients exhibited an increased HR and decreased cardiac vagal tone during rest as compared to controls.17,20,21 However, Licht et al.22 reported that HRV reductions in GAD patients are driven by medication effects alone rather than the diagnosis of GAD. The conflicting results might be due to heterogeneity in relatively small samples, confounds from medication, physical health, habitual physical activity, smoking, psychiatric comorbidities, and reporting of different HRV measures. To address these concerns as mentioned above, one should analyze with better methodology. GAD is often associated with an increased risk of developing a secondary diagnosis of major depression (MD), as suggested by epidemiological studies.23,24 For example, around two thirds of patients with a lifetime diagnosis of GAD retrospectively report MD,25 suggesting that GAD has a tendency to precede depression and finally develops into depression. However, decreased HRV can be seen in MD26 and the measures of HRV have been correlated with depressive severity, including both global26 and symptom-specific indices.26,27 Thus, factors concomitant with the MD diagnosis itself might influence the observed relationship between GAD and HRV. One might wonder whether the comorbid diagnosis of MD in GAD patients is associated with greater reduction in HRV as compared to GAD patients without comorbidity. Taken together, the following hypotheses were tested: 1) Physically healthy, unmedicated GAD patients without comorbidity will have decreased resting HRV as compared with age- and sex-matched controls; and 2) GAD with comorbid MD will display greater reductions in resting HRV as compared to non-comorbid GAD/orMD.
METHODS
Subjects
This study was approved by the Institutional Review Board for the Protection of Human Subjects at the Tri-Service General Hospital, a medical teaching hospital of the National Defense Medical Center in Taipei, Taiwan. We obtained written informed consent from all participants and fully explained the procedures of the study. Initial study entry criteria: age 20-65. After detailed questionnaire screening, clinical examination and chart review, we excluded subjects with pregnancy, smoking, diabetes, cancer, neuropathy, any cardiovascular disease that affects HRV or engaging in regular physical training exceeding 10 hours a week. Subjects who used any medication (e.g., antipsychotics, anticholinergics,antidepressants, oral contraceptives, anticonvulsants, anxiolytics, cerebral metabolic activators, or cerebral vasodilators) that have been reported to affect the ANS functioning for at least two weeks before evaluation were also excluded.
Based on the same methodology in our previous studies,26,28 each patient was evaluated using the Chinese Version of the Modified Schedule of Affective Disorder and Schizophrenia-Lifetime (SADSL)29 to reach DSM-IV criteria for a primary diagnosis of GAD. Our previous study has reported diagnostic data with satisfactory interrater reliability.30 Here, we further excluded individuals with a history of substance dependence, organic brain disease, any comorbid anxiety disorders or major psychiatric disorders other than MD. GAD patients without comorbidity were recruited in GAD group (n=20). GAD patients who met criteria for an additional current diagnosis of major depression (MD) were recruited in GAD with MD group (n=20). The severity of MD was assessed with the 17-item version of the Hamilton Depression Rating Scale (HAM-D). Only subjects with a minimum score of 18 on the HAM-D entered the GAD with MD group. We also recruited the "depression-only" group from outpatient setting. This group comprised MD patients without comorbidity (n=40), who are parts of participants described in one of our previous studies.26 Finally, control group (n=60) was recruited from the community. We used the modified Chinese Version of SADSL to exclude individuals with psychiatric conditions. Control subjects had no lifetime history of a mental disorder. Three patient groups were matched to control group on age, gender, and education.
Assessment of anxiety severity
All participants were assessed using self-report measures of anxiety, i.e., Beck Anxiety Inventory (BAI).31 The BAI contains 21 items that measure anxiety-related symptoms and was shown to have good test-retest reliability and fair concurrent validity. Subjects were asked to rate the severity of their anxiety on 4-point Likert scales (0-3). They were also assessed by an attending psychiatrist (HAC) using clinician-rated scales, i.e., the Hamilton Anxiety Rating Scale (HAM-A).32 Both the BAI and the HAM-A provide global indices of anxiety severity. To avoid multiple testing of the same hypothesis the analysis of the relationship between HRV parameters and global anxiety, severity was based on the HAM-A. The results were unchanged whether interviewer or self-reported measures of anxiety severity were used as an outcome.
Assessment of depression severity
All participants were interviewed by HAC using the 17-item HAM-D, an objective scale to assess severity of depression. In addition, we used the Beck Depression Inventory (BDI), a 21-item questionnaire, to assess subjects' self-reported severity of depression.33 The results are scored by summing the responses to each of the items in order to obtain a total depression score (range, 0-63).
Measurements of HRV
Detailed procedures have been reported previously.34 In short, after sitting quietly for 20 min, a lead I electrocardiogram was taken for 5 min while the subject lay quietly and breathed normally. An HRV analyzer (SSIC, Enjoy Research Inc., Taiwan) acquired, stored and processed electrocardiogram signals. Under a sampling rate of 512 Hz, signals were recorded using an 8-bit analog-to-digital converter. Stationary R-R interval values were resampled and interpolated at a rate of 7.11 Hz to produce the continuity in a time domain. Power spectral analysis was performed using a nonparametric method of fast Fourier transformation (FFT). The direct current component was deleted and a Hamming window was used to attenuate the leakage effect. The power spectrum was then quantified into standard frequency-domain measurements defined previously,34 which consisted of variance (variance of RR-interval values), low frequency (LF: 0.04-0.15 Hz), high frequency (HF: 0.15-0.40 Hz) and, and the ratio of LF to HF (LF/HF). All of the measurements were logarithmically transformed to correct skewed distribution.34 Vagal control of HRV is represented by HF, whereas both vagal and sympathetic control of HRV is jointly represented by LF. The LF/HF ratio is considered by some investigators to mirror sympathovagal balance or sympathetic modulations.
Statistical analyses
SPSS (version 13.0, SPSS, Taipei, Taiwan) statistical software was used for all analyses. Discrete variables in patients and controls were compared using chi-square test. Differences between continuous variables were evaluated using Student's t-test when normally distributed, otherwise the Mann-Whitney U test was used. Analyses with variances (ANOVA) with post hoc test were used to compare the HRV indices and other continuous variables in group comparisons. To evaluate relations between variables with normal distribution, we used Pearson's correlation test. For non-normal distribution Spearman's correlation test was used. The associations between HRV measures and age, body mass index (BMI) and habitual physical activity were analyzed with product-moment correlations, whereas point-biserial correlations were used to assess relationships with gender. Results of the point-biserial correlations were identical to those arising from comparisons using t tests. Linear regression analyses were used to primarily assess associations of scores of HAM-A, HAM-D with HRV indices. To control the confounding effect, we used multiple regressions on the HRV indices, with HRV-associated factors as covariables. All results are two-tailed and a probability value p<0.05 was considered statistically significant.
RESULTS
Demographics and clinical characteristics
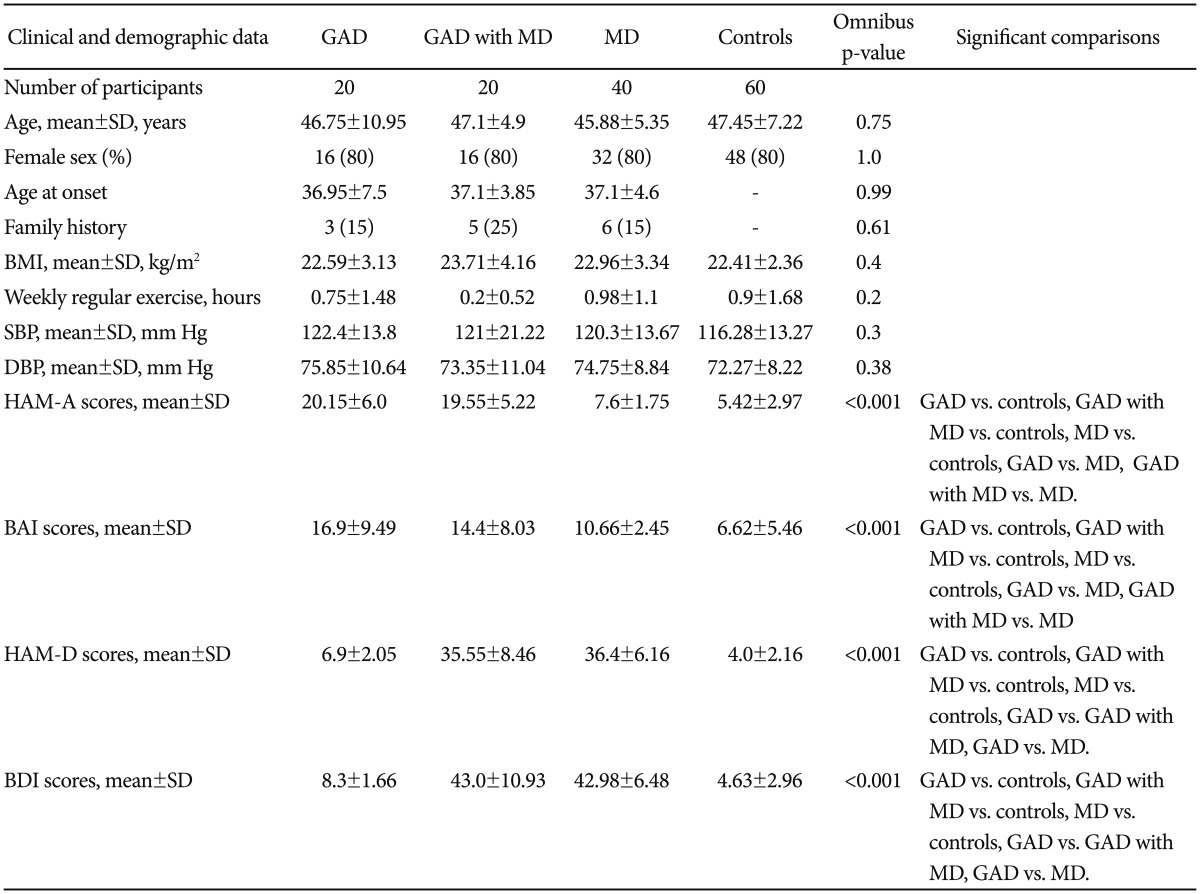
The groups did not differ significantly on demographic data, BMI, systolic/diastolic blood pressure and habitual physical activity (Table 1). Three patient groups were comparable regarding age at onset and family history. Group comparisons showed significant differences in scores of HAM-A, BAI, HAM-D, and BDI. Post hoc test revealed greater scores of HAM-A and BAI for three patient groups vs. controls, GAD group vs. MD group and GAD with MD group vs. MD group. Greater scores of HAM-D and BDI were shown for three patient groups vs. controls, GAD group vs. GAD with MD group and GAD group vs. MD group.
Heart rate variability parameters
Group comparisons showed significant differences in mean R-R intervals (RR), variance, LF and HF (all p values <0.001). However, the groups were comparable regarding LF/HF ratio (p=0.12). In Figure 1, the GAD with MD group had significantly faster HRs (shorter RR interval) than control group (782.45±143.89 milliseconds vs. 923.33±128.66 milliseconds). Post hoc test in variance and HF showed lowest values in the GAD with MD group [5.94±0.91 ln(ms2) and 3.41±1.05 ln(ms2)], greatest values in the control group [7.29±0.77 ln(ms2) and 5.48±0.89 ln(ms2)], and intermediate values in the GAD group [6.65±0.74 ln(ms2) and 4.45±1.34 ln(ms2)]. Both the GAD group and the GAD with MD group had lower LF [4.75±0.9 ln(ms2) and 4.13±1.36 ln(ms2)] than the control group [5.58±1.13 ln(ms2)].
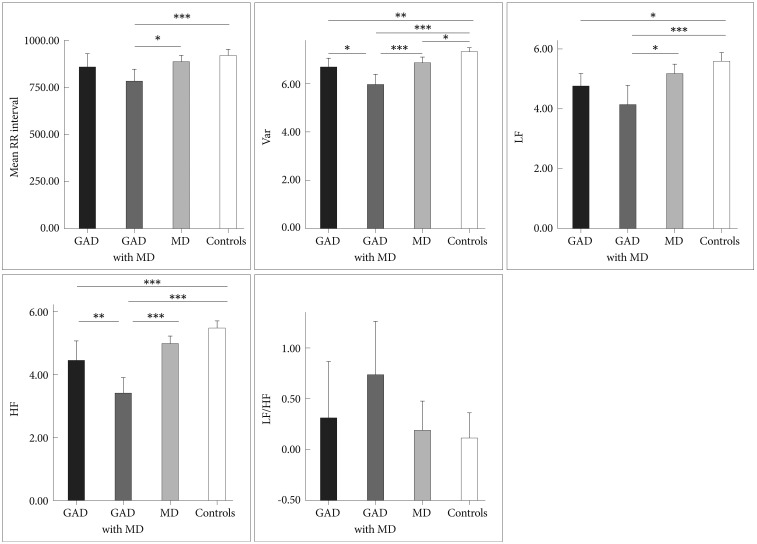
Mean R-R Intervals and all measures of HRV for GAD group, GAD with MD group, MD group and controls. Asterisks indicate significant between-groups differences. *p<0.05, **p<0.01, ***p<0.001. Var: total variance [ln(ms2)], LF: low frequency power [ln(ms2)], HF: high frequency power [ln(ms2)], LF/HF: ratio of LF to HF [ln(ratio)].
Factors associated with HRV
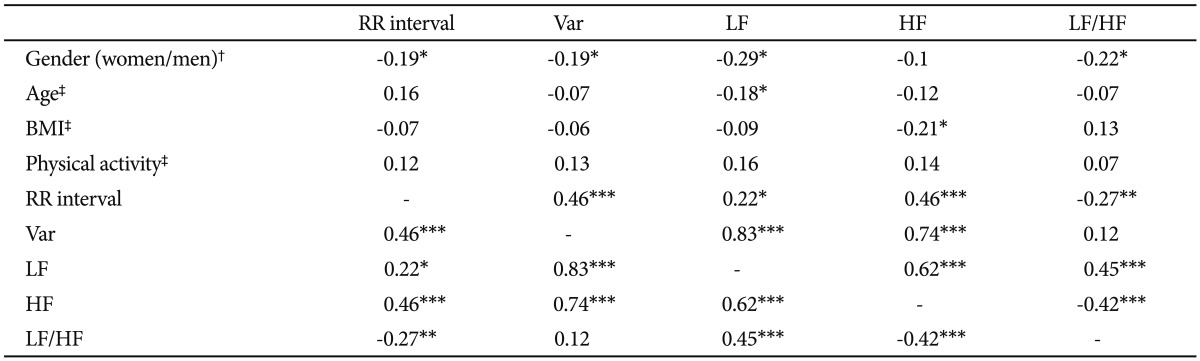
Associations between HRV measures and those potentially confounding variables are summarized in Table 2. Men had significantly faster HRs (shorter RR interval) and lower LF and LF/HF ratio than women. Participants with higher BMI had lower HF. Participants who were habitually more physically active had significantly greater LF and HF. The correlation analysis of individual HRV indices showed that variance was positively correlated with mean R-R intervals, LF and HF.
Association between anxiety/depression severity and HRV
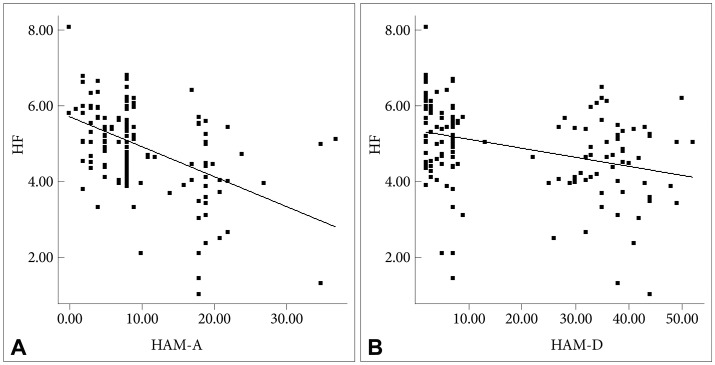
As can be seen in Table 3, subjects with greater scores of HAM-A/HAM-D had significantly faster HRs (shorter RR interval). Either HAM-A or HAM-D scores were inversely associated with variance, LF and HF. However, there was no significant correlation between scores of HAM-A/HAM-D and LF/HF ratio. Further adjustment for gender, and BMI did not alter the above-mentioned association in a meaningful way (Table 4). To analyze the specific influence of anxiety or depression severity on HRV, we performed a stepwise multiple regression with HAM-A/HAM-D scores as predictors of variance, LF and HF. We found that the HAM-A scores explained significantly the variation of HF [r2=0.234, F(1, 138)=42.22, p<0.001, b=-2.95] (Figure 2A), variance [r2=0.187, F(1, 138)=31.64, p<0.001, b=-3.47] and LF [r2=0.18, F(1, 138)=30.27, p<0.001, b=-2.55]. To a lesser extent, The HAM-D scores explained significantly the variation of HF [r2=0.113, F(1, 138)=17.6, p<0.001, b=-4.72] (Figure 2B), variance [r2=0.113, F(1, 138)=17.58, p<0.001, b=-6.2] and LF [r2=0.06, F(1, 138)=8.54, p=0.004, b=-3.34]. When separately analyzing the correlations between clinical symptoms and HRV indices among GAD patients, MD patients and controls, we only found HAM-D scores negatively correlated with HF among GAD patients. However, further adjustment for gender, age, BMI and physical activity largely reduced the β coefficients and rendered the correlation non-significant (p=0.075).
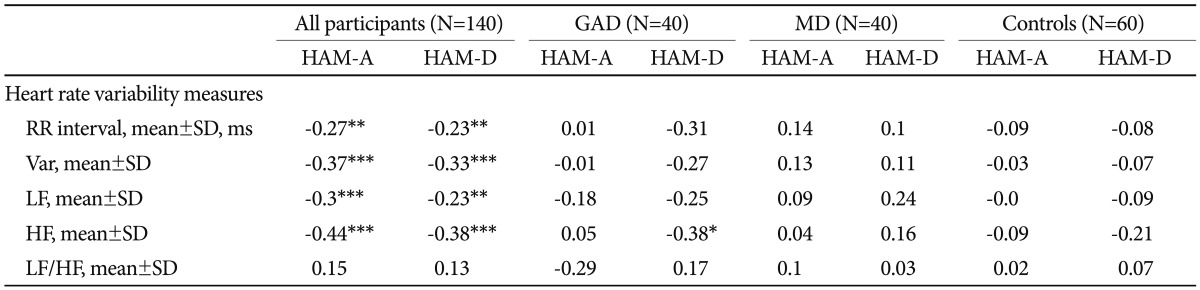
The correlations between HRV indices and scores of HAM-A and HAM-D among all participants, GAD patients, MD patients and controls, respectively
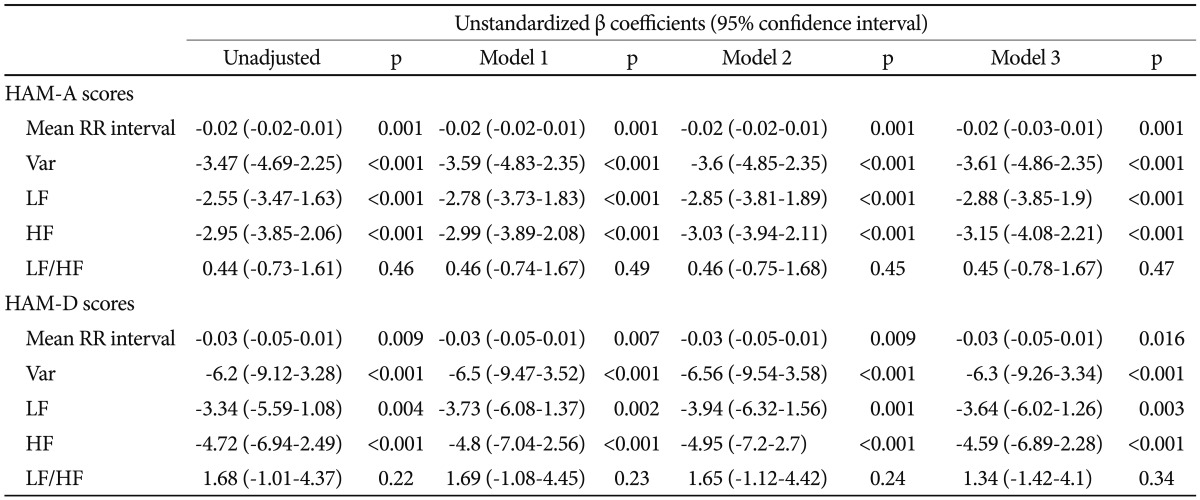
Multiple regressions of HRV parameters by scores of HAM-A and HAM-D, adjusting for gender, age and BMI factors: unstandardized regression coefficients
DISCUSSION
The main results of our study are summed as follows. First, we found physically healthy, unmedicated GAD patients had lower HRV than healthy controls. This result is consistent with previous investigations17,20 suggesting that reduction in HRV is a psychophysiological marker of GAD. However, the largest study published so far reported that GAD patients did not differ from controls in HRV and that lower HRV in current GAD was derived from the effect of antidepressants rather than the diagnosis itself.22 We believe that our result is reliable based on the following reasons. 1) Although the sample size of the study undertaken by Licht and colleagues is impressive, a number of methodological issues may account for their findings. For example, the authors only used time-domain measures of HRV. It is possible that very subtle autonomic changes accompany GAD and a time-domain analysis of HRV is insufficient to detect such small differences. Frequency-domain analysis of HRV is a sophisticated tool for the detection of the cardiac autonomic regulation and can add information on quantification of parasympathetic and sympathetic nervous system function over time-domain analysis of HRV.1,35 2) We have excluded subjects with psychiatric and physical co-morbidities that could potentially confound the association between GAD and cardiac autonomic functions. Participants in the GAD group were interviewed with the modified Chinese Version of SADSL29 to rule out any psychiatric comorbidity. Thus a false-positive result due to inclusion of depressive disorders, other anxiety disorders or substance use disorder in the GAD group is presently unlikely. 3) We have also controlled other confounding factors that may suppress or magnify the true effects of GAD on HRV, including medication, age, smoking, BMI and physical activity levels.15 For example, all our participants do not smoke or have a history of smoking. Current smoking clearly depresses HRV and, even among those who have recently quit, HRV remains lower compared with that of normal nonsmokers.36 Therefore, smoking must be taken into account in any study of the effect of GAD on HRV because a substantial proportion of GAD patients smoke or have a history of smoking.37 4) Ethnic stratification among study samples may lead to resetting population HRV patterns.38,39 For example, evidence indicated that Asian ethnicity served as significant predictors of healthy HRV as compared to European-Americans in a non-medical sample.39 Studying a genetically heterogeneous population might produce a false-positive or false-negative result by chance rather than reveal a direct relation. However, all our subjects were unrelated Han Chinese subjects drawn from a population pool in Taiwan that is known to be genetically homogeneous.41 All of the biological grandparents of our recruited subjects were of Han Chinese ancestry. Therefore, it is less likely that ethnic stratification bias produced a false-positive result in our study.
Second, correlation analysis showed a strongly positive association between variance and HF-HRV (r=0.73, p<0.001), suggesting that an overall reduction in HRV in GAD patients was at least partly derived from suppression of parasympathetic input as indicated by low HF-HRV. The following two findings further complement our result regarding lower HFHRV in GAD. 1) Patients with more severe anxiety symptoms tend to have lower HF-HRV than those with less severe anxiety symptoms. This finding is consistent with prior work reporting that GAD and its cardinal feature (worry), are associated with lower cardiac vagal control.17 2) Given the large contribution of the PNS to resting HR, findings for resting HF-HRV in any single study will theoretically parallel the findings for resting HR. This theoretical perspective seems to be supported by the fact that resting HR in GAD patients elevated on a trend level (Figure 1). However, it would be premature to make a conclusion regarding resting HR in GAD based up the preliminary result reported herein. In order to conclude GAD-related alterations in resting HR, larger samples and/or meta-analyses are needed. Taken together, our findings and can be interpreted as evidence that the basal autonomic state of GAD is characterized by decreased parasympathetic tone. The following theoretical perspectives support our findings. 1) Polyvagal theory proposed by Porges highlights the importance of the vagal pathway in attention, emotion expression, social bonding and flexible adjustment to environmental demands,41 and all of them are compromised in GAD patients.42 Vagal influences to the heart serve to dampen the sympathetic reactions to stress and to promote calm behavioral states and self-regulation.14 Without this protective function of vagal tone, subjects may become vulnerable to anxious apprehension and worry, involving pre-attentive biases to threat information, and rigid and inflexible response patterns, which characterized GAD.42 2) Another explanation proposed for the link between GAD and decreased parasympathetic tone is the inability to disengage threat detection, which serves to perpetuate hyperarousal and worry, even when no real threat exists,43 which may cause a chronic withdrawal of PNS activity and long-term reductions in HRV, which subsequently increasing the risk for CVD. In consistent with this explanation, two prospective follow-up studies on patients with stable CAD reported GAD as having a distinctly negative impact on subsequent cardiovascular events.6,7
Third, significant reduced LF-HRV in our GAD patients did not cohere with what we would have expected from other cardiac autonomic results, i.e. elevated LF-HRV.17 In addition, we failed to find a LF/HF ratio difference between GAD patients and controls, although it is hypothesized that reduced vagal modulation is accompanied by a subsequent displacement of the sympathovagal balance (as indexed by the LF/HF ratio) in favor of sympathetic modulation. These unexpected findings warrant comment. 1) The traditional interpretations of the HRV measures used in our study are that HF power estimates vagal tone, while LF power reflects both vagal and sympathetic influences. However, it has also been reported that when LF power is assessed in the supine position, administration of atropine (a potent inhibitor of parasympathetic muscarinic receptors) eliminates most of the LF region of the power spectrum.44 This does not occur when LF power is assessed in the sitting position, and suggests that resting LF power in our study may primarily reflect vagal influences.45 Consistent with this speculation is evidence that there is a 0.62 correlation between LF-HRV and HF-HRV (Table 2). 2) The LF/HF ratio was calculated from the absolute values of the LF and HF power for each subject and was considered to be an indicator of the sympathovagal balance or sympathetic modulations. However, there is mounting evidence against LF/HF ratio representing sympathovagal balance. For example, Goedhart et al.46 reported that the LF/HF ratio did not show the expected correlation to the preejection period, an established measure of cardiac sympathetic control. More specifically, Goldstein et al.47 considered LF power to be an index not of cardiac sympathetic tone but of baroreflex function during supine rest. Additional studies are recommended (e.g., cardiac noradrenaline spillover) for adequately assessing sympathetic nerve activity.
Finally, we found that HF-HRV was more reduced in GAD with MD group as compared to GAD group, and that these reductions were not due to anxiety severity - both groups rated similarly on levels of anxiety severity - or other potential confounding variables such as age, gender, BMI and physical activity. This finding is reinforced by the inverse correlation between HF-HRV and depression severity (Figure 2B), which coheres with what we would have expected from our prior study result.26 Our data provides an important extension to prior work on GAD,17,20,21 indicating that comorbid depression is associated with the greater reductions in CVC. In contrast to our findings, Hofmann et al.48 observed that GAD patients with comorbid MD had greater HF-HRV values than did those without MD. However, the authors use medicated patient sample, and therefore the influence of medication cannot be ruled out. Moreover, there was no healthy control group in that study. Lack of such a comparison with controls would have weakened the interpretability of their findings. We proposed two explanations for why HF-HRV may be reduced in GAD and in those with comorbid MD in particular. One potential explanation is the cumulative effect, i.e. combining the psychophysiological impact of the two disorders. Previous research has shown that both GA D17,21 and MD15,26 are associated with decreased CVC. A plausible scenario may be that patients with comorbid GAD and MD have greater reduction in CVC than those with only one diagnosis. As can be seen in Figure 1, the GAD with MD group had the lowest values of HF-HRV among three patient groups indeed. Also, the cumulative effect may be partly responsible for the significantly elevated HRs in the GAD with MD group (Figure 1). Another explanation is that the comorbidity between the two disorders may recognize a unique group of patients.49 Indeed, the nosological rules of DMS-IV reflect the uncertainty as to whether GAD and MD represent two separate diagnostic entities. For example, the symptoms of GAD do not occur exclusively during a major depressive episode while one can only made a diagnosis of GAD in individuals with MD if the GAD symptoms also occur outside a major depressive episode. Our data provide further evidence for the possibility that individuals with comorbid GAD and MD can be distinguished based on psychophysiological correlates (for example, HF-HRV) from individuals with GAD but without a secondary diagnosis of MD. One might argue that the differences in HRV indices between the two groups reflected difference in depression severity because HRV indices were significantly correlated with depression severity in all of our participants (Table 3). However, separate analysis of 40 GAD patients and further adjustment for HRV-associated covariables showed that HRV indices were not associated with depression severity. Taken as a whole, the between-group HRV differences did not proxy differences in severity but reflected psychophysiological differences between non-comorbid GAD and GAD comorbid with depression. Moreover, it is worth mentioning that Frasure-Smith et al. implicated GAD alone, MD alone and concurrent GAD and MD as all increasing the possibility of a poor cardiac outcome among CAD patients. However, patients with comorbid GAD and MDD were not at greater risk for major adverse cardiac events than those with only 1 factor.7 Therefore, our result must be considered preliminary. Further investigation of the link between GAD with comorbid depression, HRV and CVD is warranted.
As suggested above, our findings highlight low cardiac vagal tone in GAD patients and in those with comorbid MD in particular and should serve to remind clinicians to pay attention to their increased risk of CVD. For example, an autonomic function examination such as HRV analysis can be done to provide a rapid screening of systemic autonomic disturbance. GAD patients with comorbid depression may benefit better from cardiovascular risk reduction strategies such as exercise, smoking cessation, or undergoing treatment to restore the autonomic function. For instance, mindfulness meditation has been reported to significantly increase cardiac parasympathetic activity50 and reduce symptoms of GAD.51 Moreover, cognitive behavior therapy-based psychotherapy applied in a forest environment was helpful in the achievement of depression remission while increased parasympathetic nerve tone.52 Larger randomized control trials in GAD patients with comorbid depression to validate these study results are warranted.
Several limitations should be considered in the present study. 1) Interpretation of LF-HRV as sympathetic activity should be made with caution, as the level of modulation of LF-HRV by the sympathetic branch is still debated. 2) Our study failed to provide the information concerning the menstrual cycle of our female participants; however, HRV was shown to fluctuate during different phases of female menstrual cycle.53 3) Our study did not analyze time-domain measures of HRV, which might provide more clues regarding the different findings between our study and the study by Licht and colleagues.22 4) A recent meta-analysis emphasizes the importance of nonlinear techniques to study cardiac autonomic function and appear to be superior or of additional value to the traditional time and frequency domain measures.54 Thus, further replication with nonlinear measures is clearly desirable.
Acknowledgments
This study was supported by Tri-Service General Hospital Grant TSGHC98-91 and TSGH-C102-123 (H.A.C). The authors thank psychologist Tsuey-Yen Yeh for her assistance in the interviewing and Miss Zhang-Yu Wang for her assistance in preparing this manuscript.