Chemokine and Chemokine Receptor Polymorphisms in Bipolar Disorder
Article information
Abstract
Objective
Bipolar disorder (BD) is a debilitating psychiatric disease with unknown etiology. Recent studies have shown inflammation as a potential contributing factor of BD pathogenesis. However, potential associations between chemokine and chemokine receptor polymorphisms and BD have been fundamentally understudied. To identify participation of chemokines in BD pathogenesis, we examined genetic variants of several chemokine and chemokine receptor genes.
Methods
The study population comprised 200 patients with BD and 195 age- and sex-matched healthy controls. Genotyping of monocyte chemotactic protein 1 (MCP-1) A2518G, CCR2 V64I, CCR5 Δ32, CCR5 A55029G, stromal cell-derived factor 1 (SDF-1) 3'A, and CXCR4 C138T polymorphisms was performed using polymerase chain reaction and restriction enzyme digestion.
Results
We found that CCR5-Δ32 II and CXCR4-C138T C+ genotype frequencies contributed to an increased risk for BD. However, no statistical significance could be obtained with these genotypes after Bonferroni correction. A significant asssociation was only found with MCP-1 GG and G+ genotypes, which were markedly more prevalent in patients with BD and these genotypes seemed to significantly increase the risk for BD even after Bonferroni correction.
Conclusion
Our findings indicate an association between genetic variants of certain chemokine and chemokine receptor (especially MCP-1) genes and BD. The exact mechanisms by which these variants contribute to BD pathogenesis and their clinical implications need to be further investigated.
INTRODUCTION
Bipolar disorder (BD) is one of the most frequent and disabling conditions worldwide. Despite extensive efforts towards optimization of clinical criteria and better understanding of underlying pathogenic mechanisms, little improvement has been achieved in the treatment of BD. Interrelations between neuronal and immunologic systems have long been investigated and it has now been robustly established that cytokines and other components of adaptive immunity play key roles in host defense against microorganisms and in regulation of neurogenesis, release of neurotransmitters, control of blood-brain barrier permeability, and protection of neurons.1 It is therefore not surprising that evidence that indicates involvement of immunity and inflammation in psychiatric disorders including BD is accumulating.2
Despite their well-known involvement in neuroimmunologic processes, the pathogenic significance of chemokines, which are small signaling proteins with the ability to induce chemotaxis, has been relatively understudied in BD as compared with other immune system components. In previous studies, expression levels of several chemokine and chemokine receptors have been examined in patients with BD.1 By contrast, potential associations between chemokine and chemokine receptor polymorphisms and BD have been fundamen tally understudied and only MCP-1 gene polymorphisms have been investigated in patients with BD.3456
To further delineate the involvement of chemokines in BD pathogenesis, we compared genetic variants of several chemokines [monocyte chemotactic protein 1 (MCP-1), stromal cell-derived factor 1 (SDF-1)] and chemokine receptors (CCR2, CCR5, CXCR4) among healthy control and BD populations and identified chemokine/chemokine receptor genotypes carrying increased risk for BD development.
METHODS
Subject selection
The study sample comprised 200 patients with BD and 195 healthy control subjects with no personal or family history of psychiatric disorders. All patients had active symptoms at the time of the study. Patients and healthy control subjects were recruited from Erenkoy Psychiatric and Neurological Disorders Hospital. Each patient was given a diagnostic assessment based on clinical interviews using a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV).7 Our sample consisted of unrelated patients with BD (mean age: 40.77±11.59; males/females: 73/127), and 200 unrelated healthy controls (mean age: 42.85±10.11; males/females: 84/111).
Measurements, protocol, and procedure
The psychopathologic status of the patients was assessed by two experienced psychiatrists using the Scale for the Assessment of diagnoses in all cases were made based on case records and clinical assessments by consensus of two experienced psychiatrists according to DSM-IV criteria.7 All subjects were examined according to a standardized interview. The assessment was performed on a semi-structured sociodemographic form that required patient information regarding demographic personal details of the patients and informants, symptoms of patients, history of present illness, details of medical or surgical interventions, past history, family history, personal history, premorbid personality, details of physical examination, mental status examination, and diagnostic formulation. The diagnosis was made using the Structured Clinical Interview for DSM-IV (SCID-I).8 The patients were then screened on various rating scales such as the Brief Psychiatric Rating Scale910 for patients with BD, and the Rating Scale for Mania1112 for BD. Controls with BD were excluded from the study based on the results of the SCID-I DSM-IV interviews. Normal control participants were recruited from a large medical outpatient clinic. We investigated their demographic data, medical, and psychiatric history.
To minimize the effect of ethnic differences in gene frequencies, the study participants were recruited from the Turkish population living in Western Turkey. The study was approved by the Medical Ethics Committee of Istanbul Medical Faculty, and all participants (i.e., controls or patients) gave written informed consent.
Inclusion and exclusion criteria
Inclusion criteria were a diagnosis of current DSM-IV BD. Patients were excluded if their primary diagnosis was not BD. Patients were also excluded from the study if they had a history of neurologic or medical disorder that would affect neuropsychologic function (i.e., seizures, head trauma, stroke, brain tumor, meningitis) or if they had a recent history of abuse of alcohol or psychoactive drugs. In addition, control subjects were excluded if they had a diagnosis of any DSM-IV axis I and axis II disorders. None of the control subjects had any physical health problems.
Polymorphism analysis
DNA extraction from white blood cells was completed using the method of Miller et al.13 Polymerase chain reaction (PCR)/restriction fragment length polymorphism (RFLP) analysis was performed for the detection of the variations in these region of MCP-1 A2518G, CCR2 V64I, CCR5 Δ32, CCR5 A55029G, SDF-1 3'A and CXCR4 C138T.14151617 PCR was performed using suitable primers listed in Table 1. Products of MCP-1A2518G, CCR2V64I, CCR559029, SDF-1, and CXCR4 were further subjected to digestion with PvuII, BsaBI, SduI, MsPI, and BccI restriction enzymes, respectively. Visualization was completed using electrophoresis through a 3% agarose gel. The relative size of the PCR products was determined through comparison of the migration of a 50-1000 bp DNA molecular weight ladder (Invitrogen, Grand Island, NY, USA).
Statistical analysis
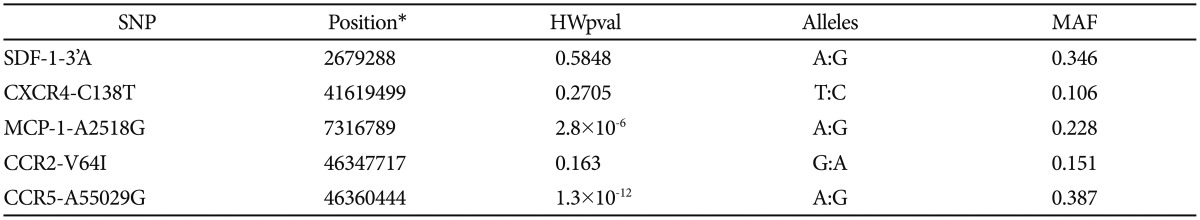
Statistical analyses were performed using the SPSS software package version 20.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as means±standard deviation (SD). Differences in the distribution of genotypes or alleles between cases and controls were tested using the Chi-square statistic, and comparison of demographic data were tested using Student's t-test. In addition, minor allele frequency and Hardy-Weinberg equilibrium were obtained using Haploview 4.2. Linkage disequilibrium among chemokine gene polymophisms was assessed using D' and r2 values obtained through the Haploview program (http://www.broad.mit.edu/mpg/haploview/documentation.php). To control multiple comparisons for 5 polymorphisms, Bonferroni correction was used and p<0.01 was considered statistically significant.
RESULTS
Demographic and clinical characteristics of subjects are presented in Table 2. Sex (p=0.218) and age (p=0.781) distribution did not differ significantly between the patients with BD and healthy controls. The genotypes were not associated with sex, initial symptoms, duration of disease, response to drugs, and clinical psychiatric tests. MCP-1 genotype distribution was significantly different between patients with BD and healthy controls (p=0.009; χ2: 9.38) (Table 3). MCP-1 G+ genotype was significantly higher in patients with BD (42%) as compared with healthy controls (29.7%) and had higher risk for BD (p=0.011, χ2: 6.44; OR: 1.70, 95% CI: 1.12-2.59). MCP-1 GG genotype incidence was significantly higher in patients with BD (13.5%) than in controls (5.6%) and increased the risk of BD (p=0.008, χ2: 7.01; OR: 2.61, 95% CI: 1.25-5.42). By contrast, MCP-1 AA genotype was significantly higher in healthy controls than in patients with BD and was associated with reduced disease risk (p=0.011, χ2: 6.44; OR: 0.585, 95% CI: 0.386-0.886). Even after Bonferroni correction, MCP-1 G+ and GG genotypes were significantly associated with increased disease risk (p<0.01).

Distribution of MCP-1-A2518G, SDF-1-3'A, CCR5-delta32, CCR5-A55029G, CXCR4-C138T and CCR2-V64I gene polymorphisms in patients with bipolar disease (BD) and healthy controls (C)
CCR5-Δ32 II genotype frequency was higher (96%) in the BD group than in healthy control group (91.8%) without reaching statistical significance and might be associated with increased risk for BD (p=0.080, Fisher's exact test, OR: 2.145; 95% CI: 0.89-5.13). Likewise, CXCR4-C138T C+ genotype frequency was higher (99.5%) in patients with BD than in controls (96.9%) without reaching statistical significance, but might be contributing to increased risk for BD (p=0.057, Fisher's exact test, OR: 6.31, 95% CI: 0.754-52.9). There were no differences with regards to CCR2-V64I, CCR5-A55029G, and SDF-1-3'A genotype frequencies between the patients with BD and the healthy control group (Table 3).
When allele frequencies were evaluated, CXCR4-C138T genotype C allele and MCP-1-A2518G genotype G allele frequencies were found to be significantly higher in patients with BD than in controls (Table 4). Nevertheless after Bonferroni correction, only MCP-1-A2518G genotype G allele frequency showed statistical significance (p>0.01).

Distribution of SDF-1-3'A, CXCR4-C138T, MCP-1-A2518G, CCR2-V64I and CCR5-A55029G allele frequencies in bipolar disease and control groups
Minor allele frequencies (MAF) are given in Table 5. With the exceptions of CCR5-A55029G and MCP-1-A2518G genotypes, most genotype distributions were found to be consistent with Hardy-Weinberg equilibrium in patients. Among the controls, only SDF-1-3'A and CXCR4-C138T genotype frequencies were consistent with Hardy-Weinberg equilibrium.
Even though GCGGA and ACGGA haplotypes were significantly higher in patients with BD than in controls and GTGGA and GTAGG haplotypes were significantly higher in controls than the patients (Table 6), these differences did not attain statistical significance according to Bonferroni correction.
DISCUSSION
To understand the underlying pathophysiology of BD, and to discover personalized markers for patients with BD, researchers have worked on several immunologic and inflammatory factors.39 These studies have brought several proteins including chemokines and chemokine-related factors to light.4041 Patients with BD present with exacerbation and remission of symptoms and may clinically be in manic, depressive or euthymic states. These clinical fluctuations are expected to affect gene expression levels. Therefore, not surprisingly, levels of the same chemokine have been found to be increased or reduced in different BD cohorts.13941 In some studies performed with patients with BD, serum levels of CXCL10, CCL3, CCL11, and MCP-1 were increased and serum levels of CCL1 and CCL22 were decreased, whereas CCL24 and CXCL8 displayed reduced or increased serum levels in different BD cohorts. In expression studies performed with quantitative RT-PCR, CCL7, CCL20, CXCL2, and MCP-1, gene expressions were upregulated and CCR2 and CX3CR1 gene expressions were downregulated on monocytes, whereas CCL3 gene expression was downregulated on brain samples of patients with BD.118192021222324 We hypothesized that polymorphism screening might give a more reflective and profound account of chemokine and chemokine receptor involvement in BD pathogenesis because polymorphism results are not influenced by clinical fluctuations.
In our polymorphism study, we found significantly increased MCP-1 GG genotype frequency in patients with BD and an increased risk for development of BD in carriers of this genotype. We also found that patients with BD displayed higher frequencies of certain chemokine/chemokine receptor alleles and haplotypes than healthy controls. Overall, our findings confirmed the involvement of chemokines in BD pathogenesis.
In previous studies, CCR5 and CXCR4 expressions have been shown to be enhanced in patients with major depression in correlation with the severity of clinical findings25 and CXCR4 expression alterations have been implicated in brain tissues of patients with BD.26 To our knowledge, our study shows an association with MCP-1-A2518G polymorphism and BD for the first time, thus shedding light on BD pathogenesis. Notably in previous studies, MCP-1-A2518G genotypes have been shown to be related with major depressive disorder but not BD.3456 Our study and previous studies on MCP-1 polymorphism displayed identical diagnostic criteria and demographic features. Therefore, this discrepancy might be due to ethnogeographic differences or different distribution of bipolar type 1 and type 2 patients.
MCP-1 levels have been found to be increased in sera, cerebrospinal fluids, and monocytes of patients with BD.1181927 Moreover, an association has been described between certain MCP-1 genotypes and depressive symptoms or suicide attempts.56 Thus, our findings together with the previous results, confer a clinical pathogenic role to this specific chemokine. There are a few potential mechanisms through which MCP-1 and other chemokine polymorphisms might influence BP development and severity. The role of inflammation in BD pathogenesis and particularly in cognitive impairment is increasingly recognized. Complex interactions between the central nervous system (CNS) pathogenic microorganism content and inflammation appear to lead ultimately to psychiatric and cognitive impairment.22829 Chemokines and chemokine receptors have important actions in the first defense of the host against viral pathogens and altered chemokine expression has particularly been associated with increased CNS viral load.3031 Moreover, MCP-1 regulates Th1 and Th2-type immune responses, which are known to be altered in patienst with BD.323334 Certain MCP-1 polymorphisms might presumably alter the functions of this chemokine, leading to increased viral load and a propensity to CNS inflammation, both of which are risk factors for BD. MCP-1 modulates neurotransmission by controlling the release of neurotransmitters and plays a neuroprotective role through its anti-apoptotic actions.353637 Most notably, there is evidence to suggest that MCP-1 adjusts the production of serotonin, a neurotransmitter closely associated with BD, by hyperpolarizing serotonergic raphe neurons in the midbrain.38 MCP-1 polymorphisms might thus affect serotonergic transmission and render susceptibility to BD development.
In conclusion, our findings indicate an association between chemokine and chemokine receptor functions and BD. The exact mechanisms by which chemokine and chemokine receptor gene polymorphisms contribute to BD development and their associations with clinical features of patients need to be further investigated.
Acknowledgments
The present work was supported by the Research Fund of Istanbul University. Project No: Normal-42079. We would like to thank Mr. David F. Chapman for English language editing.