Impact of Cannabis Use on Long-Term Remission in Bipolar I and Schizoaffective Disorder
Article information
Abstract
Objective
To investigate the impact of regular cannabis use on long-term remission of mood symptoms in bipolar spectrum disorders.
Methods
The 24-month prospective observational study included patients (n=239) with bipolar I disorder and schizoaffective disorder, bipolar type. Participants were classified as regular cannabis users (three times or more per week) or non-users. The primary outcome measure was the achievement of remission on the evaluations during the 24 months.
Results
Of the 234 participants for whom data was available, 25 (10.7%) were regular cannabis users, and the group comprised significantly more males than females. In the total population, cannabis use was significantly associated with decreased likelihood of remission during the 24-month follow-up period. Subgroup analyses showed that cannabis use was significantly associated with lower remission rates on the Hamilton Depression Rating Scale in females (n=139) and patients prescribed mood stabilizers alone (n=151), whereas in males (n=95) and patients prescribed olanzapine and/or a mood stabilizer (n=83), cannabis use was significantly associated with lower remission rates on the Young Mania Rating Scale. Remission rates were lowest in the concurrent cannabis and tobacco smoking group (n=22) followed by the tobacco smoking only group (n=97), and the non-smoker group (n=116). The post-hoc analysis revealed that all remission rates were significantly lower in the concurrent cannabis and the tobacco smoking group compared to the non-smoker group.
Conclusion
Cannabis use negatively affects the long-term clinical outcome in patients with bipolar spectrum disorders. A comprehensive assessment and integrated management of cannabis use are required to achieve better treatment outcomes for bipolar spectrum disorders.
INTRODUCTION
Cannabis use is common in psychiatric populations;12 it is a well-documented risk factor for schizophrenia and is associated with a poor prognosis for the condition.3456 Cannabis is one of the most commonly abused drugs among patients with bipolar disorder.178 Several cross-sectional studies of patients with bipolar disorder have shown that cannabis use is associated with an earlier age of onset, severe mood symptoms, greater disability, and a higher suicide risk compared with non-users.269101112 A few prospective studies have revealed that cannabis users exhibit poor adherence to treatment and have higher levels of overall illness severity, mania, and psychosis than do non-users.131415 However, the effect of cannabis on the long-term clinical course of patients with bipolar spectrum disorders have been relatively less studied, whereas several prospective studies have examined the effects of alcohol use disorders and tobacco smoking on the course of bipolar illness. 161718
Previous studies of bipolar disorder have found that cannabis use is more frequent in males than in females.81920 Moreover, gender differences in illness characteristics and course have been widely documented in psychiatric illnesses including bipolar disorder.2202122 However, to our knowledge, no study of the effect of cannabis use on long-term clinical outcomes has investigated gender differences.
Our 24-month prospective study investigated the impact of cannabis use on the clinical course of patients with bipolar spectrum disorders with a focus on long-term remission of mood symptoms according to gender and type of medication. The present study analyzed data from the Bipolar Comprehensive Outcomes Study (BCOS),23 a 2-year, prospective, naturalistic observational study designed to improve understanding of the treatment and outcomes of patients diagnosed with bipolar I disorder or schizoaffective disorder.
METHODS
Study design and participants
The rationale and design of the BCOS have been detailed previously.2324 Briefly, participants were enrolled in the study if they met the diagnostic criteria for bipolar I disorder or schizoaffective disorder, bipolar type, were aged 18 years or older, and were willing and able to comply with the study requirements. To obtain a representative sample of participants, the exclusion criteria were minimal. Participants could commence participation in the study at any phase of their illness. As this was an observational study, participants were not randomised to treatment groups but instead received their usual care, which could be varied by their treating clinician. At baseline, participants had to be receiving at least one of several conventional mood stabilizers (lithium carbonate, sodium valproate, or carbamazepine), olanzapine as a mood stabilizer, or a combination of a conventional mood stabilizer plus olanzapine. All treatment decisions were made by the participant's primary treating clinicians, independent of the study. Participants were recruited from October 2003 to May 2008 from Australian public hospitals and private clinics. Trained evaluators assessed participants on nine separate occasions: at study entry and every 3 months up to 24 months. All participants provided informed consent to participate in the study, which was conducted in accordance with Australian ethics and the Helsinki Declaration of 1975 and approved by the relevant ethics committees.
Procedures and measures
Diagnoses were confirmed by the Mini-International Neuropsychiatric Interview (MINI) version 5, a semi-structured interview designed to identify major Axis I psychiatric disorders including alcohol dependence and abuse.25 Suicide risk in the past month was also measured by six questions during the MINI interview. High suicide risk was defined by scores on the relevant questions on the MINI interview.
The primary outcome measure of this study was the rate of achieving remission over the nine evaluation points (number of remissions/9) during the 24 months. Missing data resulting from non-visits were operationally defined as indicative of non-remission. Symptomatic remission was defined as a Young Mania Rating Scale (YMRS)26 total score of ≤1227 or a 21-item Hamilton Depression Rating Scale (HAMD-21)28 total score of ≤8.2930 Total remission was defined as remission of both YMRS and HAMD-21 scores.
Our key independent variable was cannabis use. Participants were questioned about cannabis use at visit 1 using the Habits form.31 Frequency of cannabis use during the past 3 months was investigated. Participants who reported regular cannabis use 3 or more days per week were classified as cannabis users, which was consistent with previous studies.3233 The remaining participants were classified as cannabis non-users. In terms of tobacco smoking, the BCOS study classified participants as those who smoked tobacco daily and those who did not.
Statistical analysis
Sociodemographic and clinical variables of the cannabis user and non-user groups were compared using independent chi-squared tests, t-tests, or Mann-Whitney U-tests as appropriate. The effect of cannabis use on total, 21-item Hamilton Depression Rating Scale (HAMD-21), and the Young Mania Rating Scale (YMRS) remission rates during the 24-month follow-up period were compared using the Mann-Whitney U-test. Additionally, comparisons were made across gender and medication type. Spearman's correlation test was used to assess the relationship between age and remission rates. In order to explore the effect of tobacco smoking on remission rate, the participants were divided into three subgroups: concurrent cannabis and tobacco smoking, tobacco smoking only, and non-smoking. Remission rates among the three groups were compared using the Kruskal-Wallis test and post hoc paired comparisons were conducted using the Mann-Whitney U-test with the Bonferroni correction. Statistical significance was defined as p-values of ≤0.05. All statistical tests were two tailed.
RESULTS
A total of 239 participants were enrolled in the BCOS study. We were unable to obtain information on cannabis use from 5 of the 239 participants. Of the 234 participants whose data were analyzed, 213 (91.0%) completed the 24-month study. The mean age of participants was 41.8±12.7 years and 59.4% were female. A total of 172 participants were diagnosed with bipolar I disorder and 62 were diagnosed with schizoaffective disorder, bipolar type.
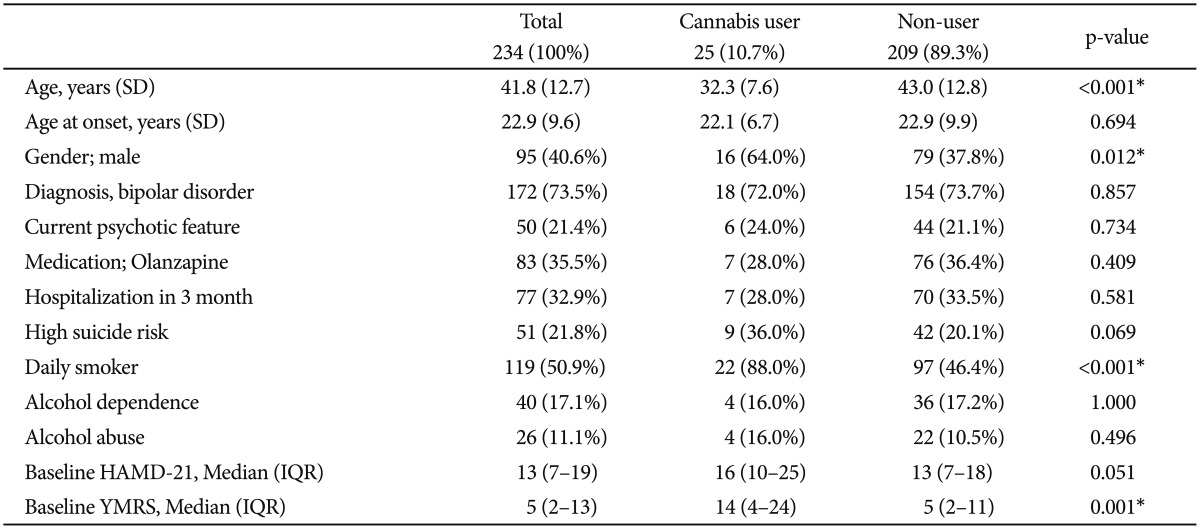
Sociodemographic and clinical characteristics were compared according to cannabis use (Table 1). A total of 25 (10.7%) subjects regularly used cannabis. The mean age of cannabis users was significantly lower than that of non-users. Significantly more males than females were cannabis users. The percentage of daily tobacco smokers was significantly higher among cannabis users than among non-users; only 3 (12.0%) regular cannabis users did not smoke daily. Baseline YMRS scores were significantly higher in cannabis users than in non-users. Baseline HAMD-21 scores tended to be higher among cannabis users than non-users; however, the difference was not statistically significant (p=0.051). There was a non-significant trend for cannabis users to be at higher risk of suicide than non-users at baseline (p=0.069). No significant difference of concurrent alcohol dependence and alcohol abuse existed between cannabis users and non-users.
The Spearman's correlation coefficients revealed no significant difference between age and total (rho=0.066; p=0.312), HAMD-21 (rho=0.065; p=0.314), or YMRS (rho=0.102; p=0.114) remission rates. Table 2 shows remission status among cannabis users and non-users according to gender and medication type. Cannabis use was significantly associated with low total remission and HAMD-21 remission rates during the 24-month follow-up period. YMRS remission rates were lower in cannabis users than in non-users; however, the difference did not reach statistical significance (p=0.051).
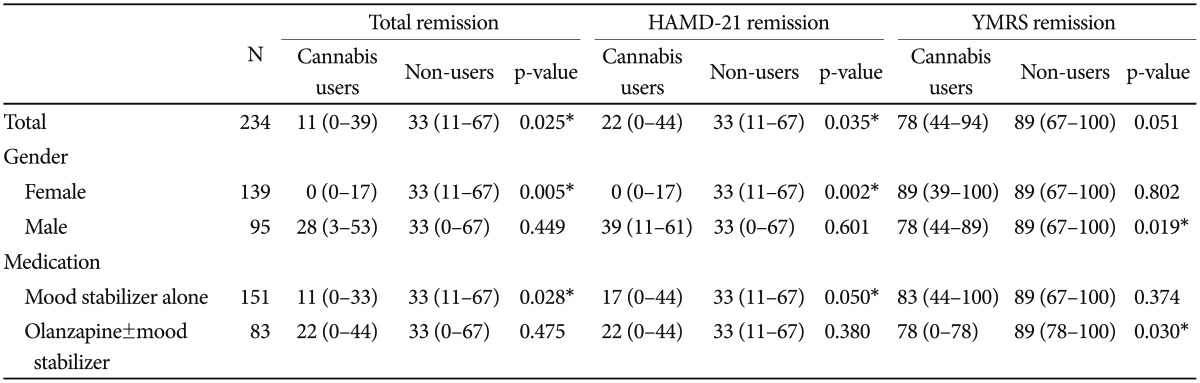
Remission rates among cannabis users and non-users according to gender and medication type during the 24-month follow-up period
The analysis of remission rates in cannabis users and non-users across gender and medication type revealed that cannabis use was significantly associated with lower total remission and HAMD-21 remission rates in females (n=139), whereas it was significantly associated with lower YMRS, but not HAMD-21, remission rates in males (n=95). Cannabis use was significantly associated with lower total remission and HAMD-21 remission rates among patients who were prescribed mood stabilizers alone (n=151); however, cannabis use was significantly associated with lower YMRS remission rates in patients who took olanzapine and/or mood stabilizers (n=83).
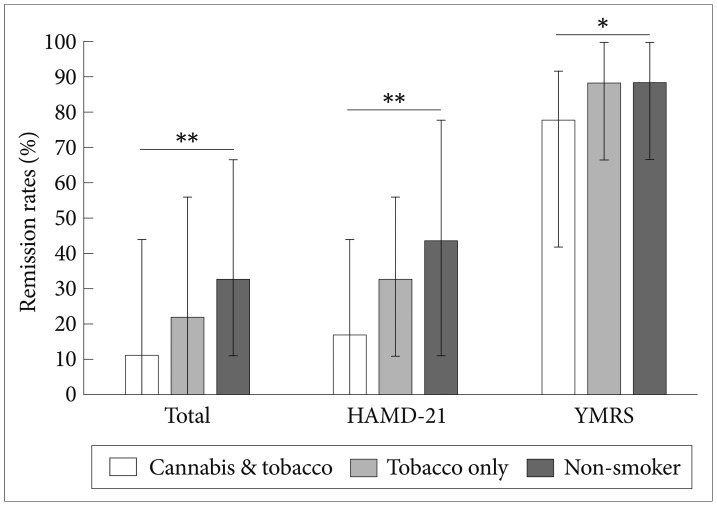
Figure 1 shows total, HAMD-21, and YMRS remission rates among the concurrent cannabis and tobacco smoking group (n=22), tobacco smoking only (n=97), and non-smoker groups (n=116) during the 24-month follow-up period. All remission rates (total, HAMD-21, and YMRS) were significantly different among the three groups (p=0.011, 0.016, and 0.012, respectively). The remission rates were lowest in the concurrent cannabis and tobacco smoking group and the non-smoker group had the highest remission rates. The post hoc analysis revealed that all remission rates (total, HAMD-21, and YMRS) were significantly lower in the concurrent cannabis and tobacco-smoking group compared to the non-smoker group (p=0.008, 0.012, and 0.009, respectively). The remission rates in the tobacco smoking only group were higher than in the non-smoker group (p=0.048-0.050), but the difference did not reach statistical significance after the Bonferroni correction (p=0.0169). The remission rates were not significantly different between the tobacco only and concurrent cannabis and tobacco groups (all p>0.08).
DISCUSSION
Our findings show that cannabis use is associated with decreased likelihood of long-term remission in bipolar spectrum disorders. In particular, we observed interaction effects of cannabis use and mood symptoms on gender and medication type. That is, cannabis use was associated with lower remission rates for depressive symptoms in females and for manic symptoms in males. Moreover, remission rates for depressive symptoms were lower in cannabis users prescribed mood stabilizers alone, whereas remission rates for manic symptoms were lower in cannabis users prescribed olanzapine. To our knowledge, this study is the first to demonstrate differing effects of cannabis use on polarity specific long-term remission rates (24-month period) according to gender and type of medication in patients with bipolar spectrum disorders.
A few studies have demonstrated an association between cannabis use and severe manic symptoms, but not depression, in patients with bipolar disorder.1314 Furthermore, cannabis use has been shown to increase the risk of manic symptoms in the general population.34 However, a recent study showed that the association between cannabis use and mood symptoms is complex and more strongly correlated with depression than mania.35 Our results revealed interactions of depressive and manic symptom remission rates on gender and type of medication.
Our finding of an association between cannabis use and lower remission rates for depressive symptoms in females supports a previous study showing that cannabis use in teenage girls, but not boys, predicted high rates of depression.36 Moreover, cumulative evidence suggests that cannabis use increases the risk for unipolar depression.3738 This association may be linked to the greater vulnerability of women to developing depression.3639 Childhood sexual abuse, which is widespread among female cannabis users with bipolar disorder,12 is associated with poor outcomes for depression and should be considered a contributing factor to the effect of cannabis use on clinical outcome.4041 Similarly, abuse is linked to the development of maladaptive cognitive schemas and extreme attributions, which too are risks for poorer outcomes.4243
Cannabis may diminish the effect of medications used to treat bipolar spectrum disorders. Our results showed that olanzapine was associated with lower remission rates for manic symptoms in patients with bipolar spectrum disorder who were cannabis users. Investigations into the effect of cannabis on the inhibition and induction of human liver cytochrome P450 (CYP450) isoforms generally reflect a low risk of clinically significant drug interactions with most use, although in vivo studies in humans have not been conducted. 4445 However, smoked cannabis and tobacco induce hepatic CYP 1A2 enzyme activity; thus, the systemic effects of CYP 1A2 substrates, such as olanzapine, may be decreased in individuals who smoke cannabis.44454647 Furthermore, poor adherence to medications, which is common among cannabis users in bipolar populations, may contribute to unfavorable outcomes. 15484950
A high rate of co-use of cannabis with tobacco smoking and alcohol use has been observed in patients with bipolar and other psychiatric disorders.21516325152 A recent study found that co-occurring cannabis use disorder and nicotine dependence was associated with a higher prevalence of bipolar disorder compared with cannabis use disorder alone.53 In our study, alcohol dependence and abuse were not associated with cannabis use. However, cannabis use was highly associated with tobacco smoking. Previous studies, including the BCOS, have found that tobacco smoking is associated with poor mental health outcomes among patients with bipolar spectrum disorder.171854 Our results revealed a tendency for lower remission rates in tobacco only smokers compared with non-smokers. Furthermore, our findings suggest that concurrent cannabis and tobacco smoking has a cumulative effect on unfavorable outcomes in patients with bipolar spectrum disorders. Ideally, a comparison of cannabis users who did and did not smoke tobacco would help differentiate the effects of tobacco and cannabis smoking on remission rates. However, the number of cannabis users in our study population who did not smoke tobacco (n=3) was too small to perform a statistical analysis. Further studies that include subjects who smoke cannabis but not tobacco are warranted.
Substance use disorders including cannabis use often go unrecognized or are viewed as a secondary problem in psychiatric treatment settings.55 However, our results, showing a negative impact of cannabis use on the clinical course of patients with bipolar spectrum disorders, highlight the need to actively assess and manage cannabis use problems. A comprehensive evaluation of patients with bipolar disorders (particularly males) should include a systematic assessment of substance use problems including cannabis use.2 Furthermore, specialized treatment approaches should be available for this vulnerable population including motivational interview and cognitive behavioral therapy for co-occurring bipolar and cannabis use.55 Further study is required to investigate the impact of effective management of cannabis use on the clinical course of patients with bipolar spectrum disorders. In addition, the biological substrates associated with comorbid cannabis use in bipolar spectrum disorders should be investigated to clarify the mechanisms underlying the negative impact and to develop effective and safe treatment strategies.
Our study has several limitations that should be considered when interpreting our results. First, cannabis use was investiinvestigated only cross-sectionally and there was no longitudinal evaluation. Regular checks on cannabis use in patients with bipolar spectrum disorders would provide more information about longitudinal outcomes. In addition, the investigation of the relative sequence of cannabis use onset and bipolar disorder onset would provide a deeper understanding of the complex associations between these two conditions. Second, the potential impact of concomitant psychosocial therapies on the outcomes was not evaluated in this study. Third, HAMD-21 and YMRS scores were used to evaluate symptoms in the week prior to the assessment visit. Given the fluctuating course of symptoms, this timing may not adequately reflect the symptoms across the 3-month interval between visits. However, the 2-year prospective observation period provided an extended period of observation that enabled examination of the fluctuating course of illnesses and outcomes. Finally, our sample and setting might have resulted in selection bias and unmeasured confounding variables. In addition, the quality of treatment was not controlled strictly in this study. However, our prospective, pragmatic study design, high 2-year retention rate, and inclusion of patients at all levels of symptom severity and treatment allows for broad generalizability.30
In conclusion, regular cannabis use has a negative effect on the clinical course of bipolar spectrum disorders. Individuals with bipolar spectrum disorders who are regular cannabis users are a vulnerable population.9 Given the clinical significance of cannabis use in bipolar spectrum disorders, routine comprehensive and regular assessments of cannabis use are recommended. Furthermore, special attention to, and management of, patients with these substance problems must be integrated into the treatment strategy to achieve better therapeutic outcomes for bipolar spectrum disorders.
Acknowledgments
The Bipolar Comprehensive Outcomes Study (BCOS) was funded by an unconditional research grant provided by Eli Lilly Australia. Lilly had no role in the collection, analysis, interpretation of data, the writing of the report, and in the decision to submit the paper for publication.
SWK is supported by Research Institute of Medical Sciences, Chonnam National University, Korea. LB is supported by an Alfred Deakin Postdoctoral Research Fellowship. PBF is supported by an NHMRC Practitioner Fellowship (606907). MB is supported by a NHMRC Senior Principal Research Fellowship (1059660).