Effect of Probiotic Adjuvant Therapy on Improvement of Clinical Symptoms & Interleukin 6 Levels in Patients With Schizophrenia
Article information
Abstract
Objective
This study aims to examine the effect of giving probiotic adjuvant therapy on improving clinical symptoms & IL-6 levels in patients with schizophrenia.
Methods
This research was a double-blind, placebo-controlled trial conducted at Dadi Psychiatric Hospital, South Sulawesi Province, Indonesia in November–December 2021. The sample of the research was patients with schizophrenia undergoing hospitalization who received therapeutic doses of risperidone with a total of 21 samples in each treatment and control group. Research subjects were measured with Positive and Negative Syndrome Scale (PANSS) at baseline, 2nd, 4th, and 6th weeks. The treatment group received one capsule/12 hours/oral of probiotics for six weeks and the control group received 1 capsule/12 hours/oral placebo for 6 weeks. In addition, two measurements of IL-6 using enzyme-linked immunosorbent assay were performed in both groups, namely at the beginning of week 0 and the end of the 6th week.
Results
We found the decrease in the PANSS value which described the improvement in clinical symptoms of the schizophrenic group after receiving therapeutic doses of antipsychotics and probiotic capsules or the treatment group as well as the schizophrenia group receiving therapeutic doses of antipsychotics and placebo capsules or the control group.
Conclusion
Improvements in clinical symptoms and decreased levels of IL-6 in the group of patients with schizophrenia who received risperidone with probiotic adjuvant therapy were better than in the group of patients with schizophrenia who received risperidone without probiotics as adjuvant therapy.
INTRODUCTION
Schizophrenia is a complex brain disorder with an estimated lifetime prevalence of 1.6–12.1 per 1,000 people. The cause of schizophrenia has not been fully defined, but the available evidence supports the interaction of genetic and environmental variables as an etiology. Gatrointestinal comorbidity in mental illness has been described so far, with laxatives and emetics being offered as the dominant treatment strategy in the previous literature. Some recent studies reported extensive inflammatory changes throughout the gastrointestinal tract of patients with psychiatric symptoms [1].
Recent studies suggest a link between gut microbiota and mental health. Naufal et al. [2] found the occurrence of changes in stress response in experimental animals. There are several similar studies conducted. The recent findings found that the gut-brain axis affects the endocrine, neural, and immune systems [2]. The brain-gut axis is the point of action of probiotics on psychiatric symptoms. Probiotics have been shown to improve intestinal barrier function, or in the immune system, to activate regulatory T cells and improve defecation and metabolic disorders [3].
Various methods of treating psychiatric disorders have been developed. The latest treatment concept is probiotics which have psychobiotic properties. Psychobiotics are probiotics that contain psychotropic properties and proven probiotics with proper administration can provide physiological and psychological benefits for healthy people. In recent years, many interventions have been carried out to identify this effect using human and animal samples [4]. Probiotics may have beneficial effects on mood based on clinical trials [5].
Giving probiotics can potentially be useful for overcoming negative symptoms in patients with schizophrenia. The administration of probiotic supplements has shown to have a sustained effect on the gut microbiota and probiotics have shown anti-inflammatory and immune-modulating properties, and it is hoped that the administration of probiotics can improve the quality of life of patients with schizophrenia [6]. Evidence suggesting possible changes in the microbiota in schizophrenia include structural damage to the gastrointestinal tract, increased immune responses to infectious pathogens and food antigens as well as known differences in the microbiota in other neuropsychiatric disorders [4].
A person’s mental health is related to eating habits and brain health. Recent findings have revealed that food plays a major role in regulating stress and mental health. In this sense, probiotics are beneficial microbes that are claimed to provide health benefits when consumed in adequate amounts. Probiotics alter the microbial composition of the gut positively. Most studies suggest that the consumption of probiotic formulations improves cognitive function, stress management, and decision making. There is a journal that reviews recent findings regarding the effect of probiotic supplementation on cognitive function, especially in human subjects. The role of probiotics in maintaining healthy gut microbiota and detailed results from the clinical trials here were reported with an easyto-understand concept. However, research involving clinical trials is still very limited on probiotics, especially in patients with schizophrenia. This study aims to examine the effect of giving probiotic adjuvant therapy on improving clinical symptoms & IL-6 levels in patients with schizophrenia at the Hasanuddin University Hospital and its network [7].
METHODS
Research design
This research was a double-blind, placebo-controlled trial conducted at Dadi Psychiatric Hospital, South Sulawesi Province, Indonesia in November–December 2021 (Makassar, Indonesia). This research had received ethical approval from the Ethics Committee for Biomedical Research in Humans, Faculty of Medicine, Hasanuddin University with number (754/UN4.6.4.5.31/PP36/2021). All patients and families signed informed consent forms prior to inclusion in the study.
Sampling
Research participants were selected by consecutive sampling. The sample size for this study was determined in two groups of treatment and control with measurements using the following formula:
n1, number of subjects of the first group; n2, number of subjects of the second group; α, type I error, which is 5%, onesided hypothesis; Zα, standard deviation for α of 5%=1.64; β, type II error, which is 20%; Zβ, standard deviation for β of 20%=0.84; χ1–χ2, the least significant difference=1.8; s, standard deviation=18.
From this formula, the minimum sample size is 20 for each group. In this study, we used 25 subjects for each group, so that a total of 50 participants were selected for this study.
Participants
Inclusion criteria were patients with schizophrenia based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition and receiving antipsychotics according to the therapeutic dose in the form of Risperidone 4–6 mg/day, while being hospitalized, aged 20–45 years, and willing to participate in the study. Exclusion criteria used were an abuse of psychotropic drugs, alcohol and narcotics, receiving anti-inflammatory drugs or steroids and antibiotics, severe physical illness and other diseases based on history. Drop criteria were research subjects who were unable to continue the study because they were discharged from the hospital before six weeks or passed away.
Intervention
Fifty subjects were recruited and then divided into two groups. The treatment group, namely schizophrenia with risperidone and the addition of one capsule/12 hours/oral probiotic for six weeks consisted of 25 subjects, and the control group, namely schizophrenia with risperidone and the addition of one capsule/12 hours/oral placebo for six weeks consisted of 25 subjects. Clinical symptoms were measured based on Positive and Negative Syndrome Scale (PANSS) at the initial week (baseline), 2nd week, 4th week, and 6th week. Blood samples were also taken to measure the levels of IL-6 in the blood in the initial week (baseline) and at the end of the sixth week.
Objective criteria
IL-6 levels were measured in the form of serum which was duplicated by the enzyme-liked immunosorbent assay (ELISA) method from the special IL-6 kit. The examination was carried out at the Hasanuddin University Medical Research Center (HUMRC) Laboratory. The examination followed the instructions according to the Human IL-6 ELISA Kit 96 cat protocol No. E0090Hu Bioassay Technology Laboratory, Shanghai, China. Blood samples were taken at the beginning of the study and the end of the 6th week.
The PANSS is to measure positive and negative symptoms in people with schizophrenia. This instrument has several advantages, including a clearer operational method, a more comprehensive symptom assessment, a more standardized scoring determination, and has been validated in Indonesia. PANSS consists of 33 items, each of which is rated on a 7-point scale. The officers who judged were doctors who previously had a perception equation and the assessment was not carried out by the previous studies [8].
Safety
Possible side effects and complications with the administration of probiotics may occur during the study. So far, the administration of one capsule of probiotics/12 hours/orally/day is well tolerated, and no clinical side effects have been detected.
RESULTS
Characteristics of research subjects
This study began by screening the group diagnosed with schizophrenia who were hospitalized at the Hasanuddin University Hospital and its network from November to December 2021. Then after getting subjects who entered the inclusion criteria and who agreed to participate in the study, the subjects were divided into two: treatment and control groups. The initial subjects were 50 participants and divided into two groups. The control group was 25 subjects and the treatment group was 25 subjects. For each group, initial measurements were carried out using psychometrics in the form of initial PANSS and initial blood sampling for IL-6 examination. Then the treatment group was intervened using probiotic capsules at a dose of one capsule per 12 hours and the control group was intervened using a placebo capsule at a dose of one capsule per 12 hours. After taking the initial PANSS scores, then it was continued by the next PANSS examination in the second, fourth, and sixth weeks in each group. Each group had 25 subjects per group who were willing to take part in the study. Then when the study took place, eight subjects dropped out in the first week, three subjects dropping out in the second week, three subjects in the third week and two subjects in the fourth week until the end of the study in the sixth week, only 21 subjects were found. Subjects who dropped out consisted of five participants who were picked up by their families because their area of origin was quite far from the research hospital and three participants who suddenly left the hospital without the officer’s knowledge. Group subjects were then continued with the final PANSS examination in the sixth week and blood collection for examination of IL-6 levels. Characteristics of research subjects from primary data can be seen in Table 1.
The results showed that the mean age of the research subjects was 33.57±8.07 years in the treatment group and 35.71±6.41 years in the control group with more males than females. The basic characteristics of the research subjects including age, sex, education, occupation, marital status, antipsychotic dose per day (p=0.072), antipsychotic equivalent dose (p=0.076), and length of stay were not found to be significantly different between the treatment and control groups. Thus, the research subjects were homogeneous (Table 1).
Characteristics of research subjects were based on nutritional status including weight, height, and body mass index (BMI). The results showed that the weight had a p-value of 0.682 for treatment and 0.096 for control, while the height had the p-values of 0.427 for both treatment and control. Lastly, the measurement found p-values of BMI was 0.656 for treatment and 0.100 for control. As there is no significant difference between the treatment and control groups, thus the research subjects were homogeneous (Table 2). The results of the comparison of nutritional status between the treatment and the control groups found that all of the variables were not found to have a significant difference. The p-values for weight were 0.704 (baseline) and 0.630 (the 6th week), while the p-value for height was 0.427. Meanwhile, the p-values for BMI were 0.422 for baseline and 0.369 for the 6th week.
Comparison of IL-6 levels
A total of 42 study subjects who received risperidone in both the treatment group and the control group were measured serum IL-6 levels at the baseline and the end of the 6th week. The measurement results are shown in Table 3.
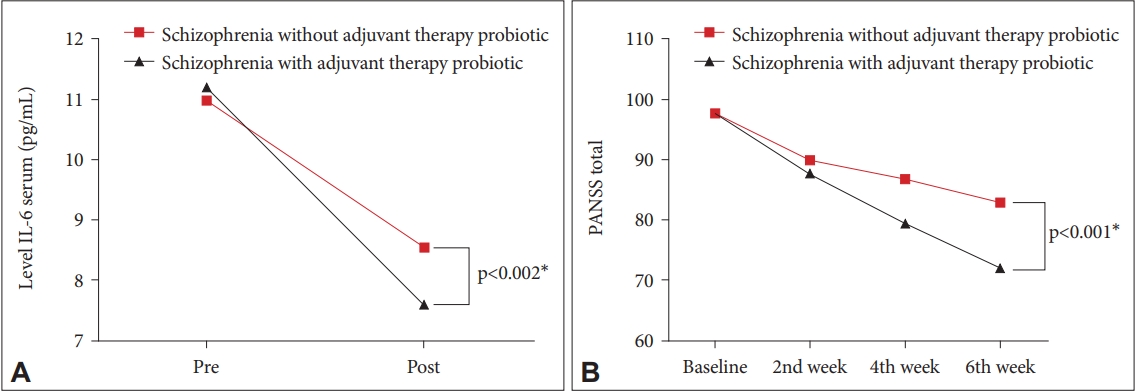
The results as shown in Table 3 found that there was a decrease in baseline IL-6 levels in the treatment group from 11.20 pg/mL to 7.59 pg/mL after 6 weeks with a difference of 3.61 pg/mL (p=0.001), while the control group on the baseline IL-6 from 11.00 pg/mL to 8.54 pg/mL with a difference of 2.46 pg/mL (p=0.001). The results in Figure 1 showed a significant decrease in IL-6 levels in the treatment group and the control group. Moreover, the decrease in IL-6 levels was greater in the treatment group than in the control group (p<0.002). This indicates a significant improvement in IL-6 levels in the treatment group compared to the control group.
Total Positive and Negative Syndrome Scale value comparison
Table 3 also showed the comparison of total PANSS values in the treatment group and control group measured at the initial week, 2nd week, 4th week, and 6th week. Based on bivariate analysis using Friedman’s test, the total PANSS values showed a significant difference between the initial week, 2nd week, 4th week, and 6th week in the treatment group (p=0.001). Meanwhile, in the control group, the total PANSS scores were compared between the initial week, 2nd week, 4th week, and 6th week which also showed a significant difference (p=0.001). Furthermore, the results obtained that the level of improvement in clinical symptoms based on the difference between the baseline and 6th week PANSS in the control group and the treatment group found a significant decrease (p=0.001) with moderate improvement in the treatment group and minimal improvement in the control group (Supplementary Table 1 in the online-only Data Supplement).
The results found a decrease in the PANSS value which describes the improvement in clinical symptoms of the schizophrenic group after receiving therapeutic doses of antipsychotics and probiotic capsules or the treatment group as well as the schizophrenia group receiving therapeutic doses of antipsychotics and placebo capsules or the control group. However, the decrease in the schizophrenia group after receiving therapeutic doses of antipsychotic and probiotic capsules was more significant than in the group that did not receive probiotics (p<0.001) (Figure 1).
Correlation between IL-6 levels and total Positive and Negative Syndrome Scale values
The relationship between IL-6 levels and the total PANSS value for the two groups can be seen in Table 4.
The results of correlation analysis using the Spearman correlation test showed the correlation between the PANSS with baseline IL-6 levels in the treatment group was not significant in the initial week (p=0.624), 2nd week (p=0.579), 4th week (p=0.598), and 6th week (p=0.343) with a weak correlation with the positive directions at the initial week, 4th week, and 6th week and negative direction 2nd week. The results of correlation analysis also showed the correlation between the PANSS with 6th-week serum IL-6 levels in the treatment group was insignificant in the initial week (p=0.863), 2nd week (p= 0.413), 4th week (p=0.995), and 6th week (p=0.912) with a weak correlation with the negative directions at the initial week, 2nd week, and 4th week and positive direction at 6th week.
The results of correlation analysis using the Spearman correlation test showed the correlation between the PANSS with baseline IL-6 levels in the control group was not significant in the initial week (p=0.357), 2nd week (p=0.682), 4th week (p=0.280), and 6th week (p=0.660) with a weak correlation with the positive directions at the initial week, 2nd week, 4th week, and 6th week. The results of correlation analysis also showed the correlation between the PANSS with 6th week serum IL-6 levels in the control group was not significant in the initial week (p=0.333), 2nd week (p=0.973), 4th week (p=0.975), and 6th week (p=0.287) with a weak correlation with the negative directions at the initial week and 6th week and the positive directions at 2nd week and 4th week (Figure 2).
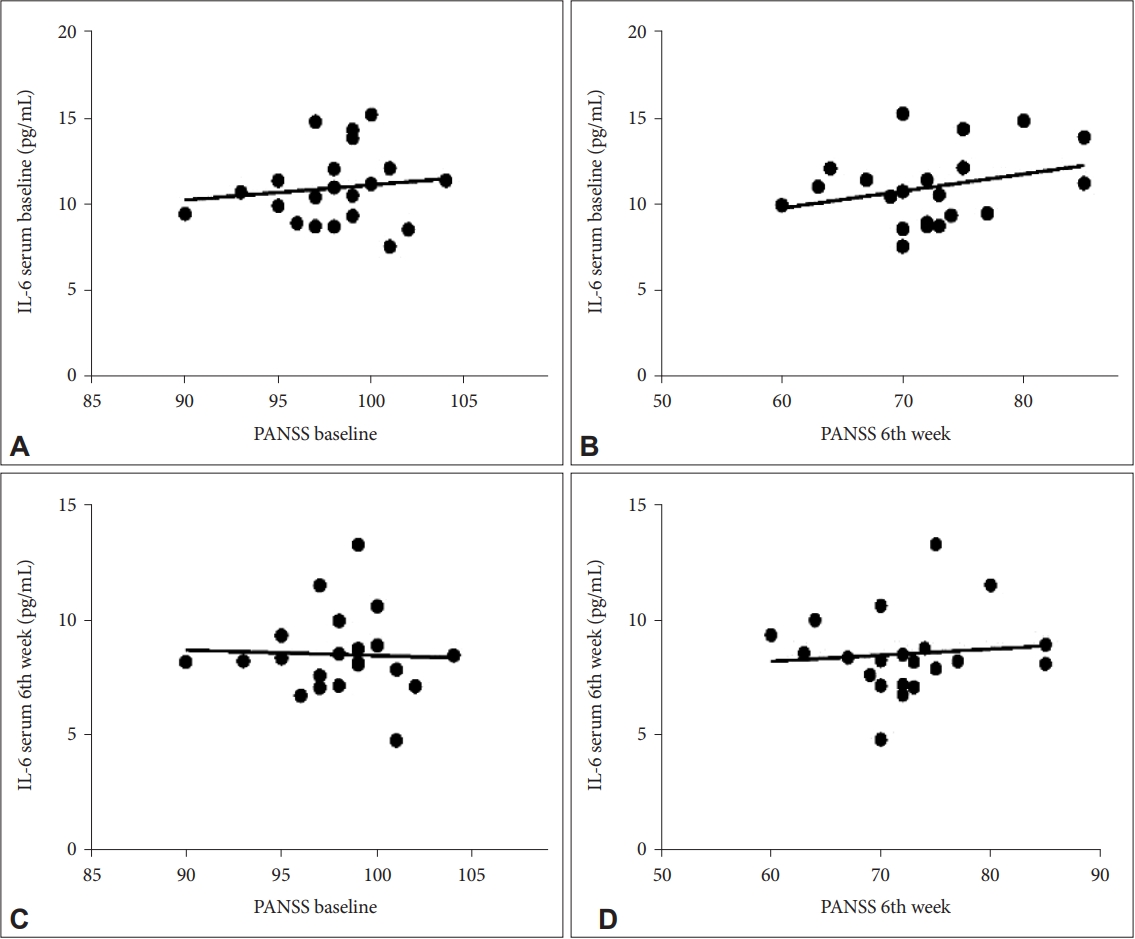
Correlation of baseline serum IL-6 levels with total PANSS at (A) baseline and (B) after 6 weeks in the treatment group; correlation of 6th week serum IL-6 levels with total PANSS at (C) baseline and (D) after 6 weeks in the treatment group. PANSS, Positive and Negative Syndrome Scale.
Furthermore, the results about the correlation between IL-6 levels and improvement in clinical symptoms (total PANSS value) in the treatment group can be seen in Figure 3A (for baseline IL-6 with total PANSS at the start of the week), Figure 3B (for baseline IL-6 with total PANSS after 6th week), Figure 3C (for 6th week serum IL-6 after with total PANSS in the 1st week), and Figure 3D (for 6th week serum IL-6 with total PANSS after 6th week).

Correlation of baseline serum IL-6 levels with total PANSS at (A) baseline and (B) after 6 weeks in the control group; correlation of 6th week serum IL-6 levels with total PANSS at (C) baseline and (D) after 6 weeks in the control group. PANSS, Positive and Negative Syndrome Scale.
Meanwhile, the results about the correlation between serum IL-6 levels and improvement in clinical symptoms (total PANSS value) in the control group can be seen in Figure 3A (for baseline IL-6 levels with total PANSS at the initial week), Figure 3B (for baseline IL-6 levels with total PANSS after 6th week), Figure 3C (for 6th week serum IL-6 levels with total PANSS at baseline), and Figure 3D (for 6th week serum IL-6 levels with total PANSS after 6th week) (Figure 3; Supplementary Table 2 in the online-only Data Supplement).
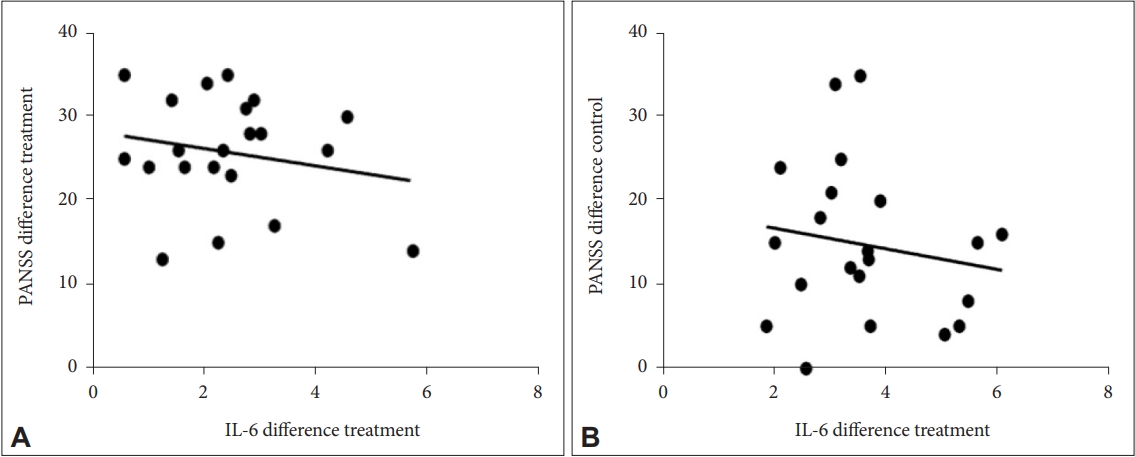
The correlation between the difference in PANSS with the difference in IL-6 was found to be insignificant in the treatment group (p=0.383) and the control group (p=0.480) with a weak correlation and negative direction (Figure 4).
DISCUSSION
This study was conducted to examine the effect of adjuvant therapy with probiotics on clinical symptoms as measured by PANSS and the effect on blood levels of IL-6 in patients with schizophrenia who were administered for 6 weeks. The basic characteristics of the research subjects including age, sex, education, occupation, marital status, antipsychotic dose per day, antipsychotic equivalent dose, and length of stay were not found to be significantly different between the treatment and control groups. Thus, the research subjects are homogeneous.
In this study, there were more male subjects than female subjects due to the distribution of men who were temporarily hospitalized during the study, more were hospitalized at the hospital where the study took place. According to theory, the prevalence of schizophrenia in males and females is the same. However, there are also differences in the onset and course of the disease. Earlier onset is usually found in males than females so that more than half of people with schizophrenia are men but only a third are women [9].
Several hypotheses can explain sex differences in schizophrenia. The theory of schizophrenia involves gonadal hormones, such as estrogen, which plays a neuroprotective role in preventing the pathology of schizophrenia in women. Estrogen deficiency at menopause is strongly associated with the severity of psychiatric symptoms in women. A negative correlation between patients’ plasma estrogen levels and schizophrenia symptoms was also reported in men. Gender-specific associations of certain dopaminergic genes (catechol-O-methyltransferase, monoamine oxidase) are also associated with schizophrenia. Meanwhile, dopamine deficits and excesses have been associated with positive and negative symptoms of schizophrenia in general [10].
Based on the highest educational qualification of patients with schizophrenia, it was almost balanced between the treatment group and the control group. In the last education treatment group, the highest number was the secondary school with 38% each and the lowest was elementary education with 23.8%. For the control group, it was almost the same as the last education level in the treatment group, the most being secondary school with 38% each and the lowest was the bachelor level with 4.76%. If it is seen from these data, individuals with schizophrenia are also less likely to have a higher education than individuals without schizophrenia, only one person has reached the level 1 education level. Poor academic achievement before the age of 16 years associated with the appearance of prodromal symptoms may represent a premorbid cognitive marker that is susceptible to schizophrenia later in life [11].
In this study, 42.8% of the research subjects did not work in both the treatment and control groups. This could be due to a lack of economic support that could make the subject enter hospitalization or the subject unable to work due to the illness he was suffering from. Previous research concluded several criteria of job function that need to be considered in people with schizophrenia, namely the work is paid or not, parttime or full time. Most people with schizophrenia often work without pay at home. However, 10%–20% of people with schizophrenia can also work competitively, where the job requires a normal recruitment process and is not part of a job program created by a social company such as job training for people with mental disorders. People with schizophrenia who are employed in normal jobs, generally get laid off because of dissatisfaction from the company with the results of the work they do [12]. Factors of stigmatization and discrimination from society cause patients to lack self-motivation and limit their right to get a job. The prognosis for patients who are unemployed and economically unstable has a worse response [13]. People who do not work will more easily experience stress associated with high stress which will eventually lead to helplessness. Some patients with schizophrenia cannot work because of difficulties in getting job opportunities and the rest decide to quit their jobs because of their illness because people who work have a sense of optimism about the future and have greater enthusiasm for life than those who do not work [14].
Based on marital status, most of the subjects were married and had partners of 13 participants for the treatment group and 17 participants for the control group. For those who are not married and do not have a partner about 8 participants for the treatment group and 4 participants for the control group. This data when compared with several previous studies is better because subjects who are married or temporarily have a partner more than those who are not married or do not have a partner. Meanwhile, another study states that a person who is not married may be at greater risk of experiencing mental disorders of schizophrenia when compared to someone who is married, although statistically no significant difference was found between marital status and the incidence of schizophrenia. Sadock et al. [9] said that the prognosis of patients with schizophrenia who are unmarried, unmarried, divorced, or widowed tends to be worse than patients with schizophrenia who have a supportive partner for the patient’s recovery [15].
The dominant dose of antipsychotic in this study was risperidone 4 mg for both groups. Only three study subjects in the treatment group received additional therapy with clozapine 25 mg. Clozapine 25 mg was given to some subjects only as a sedative therapy and did not meet the therapeutic dose as an antipsychotic treatment for patients with schizophrenia. So the equivalent dose of antipsychotic obtained for the treatment group was 405.36±13.45 mg while for the control group 400.00±10.68 because no one received treatment other than risperidone.
For assessment of nutritional status, there was no significant difference between the control and treatment groups in the form of weight (p=0.682 for treatment and 0.096 for control), height (p=0.427 for both treatment and control), and BMI (p=0.656 for treatment and 1.00 for control) at the beginning of the study until the end of the study after six weeks. For both treatment groups, there was an increase in body weight but not significant, while for the control group there was no weight gain. In previous studies, some atypical antipsychotics can stimulate weight gain. In addition, another study explained that the use of atypical antipsychotics for at least four weeks can cause weight gain in patients with schizophrenia and doctors are recommended to monitor metabolic side effects due to the use of atypical antipsychotics [16].
Several factors may alter the microbiome such as infection, disease and diet. Turnbaugh et al. [17] demonstrated that switching from a low-fat, polysaccharide-rich diet to a high-fat, high-sugar diet shifts the structure of the microbiota over the course of a day and alters the representation of metabolic pathways in the microbiome, which may occur in hospitalized patients. Recent studies have shown that antipsychotics can also alter the microbiota. There are also markers of the metabolic syndrome occurring more frequently in patients with schizophrenia receiving atypical than typical combination therapy [18].
The 42 study subjects who received risperidone in both the treatment and control groups were measured for serum IL-6 levels at the baseline and the end of the 6th week. There was also a decrease in baseline IL-6 levels in the treatment group after six weeks with a difference of 3.61 pg/mL (p=0.001), while the control group on the baseline IL-6 with a difference of 2.46 pg/mL (p=0.001). There was a significant decrease in IL-6 levels in the treatment group and the control group. This indicates an improvement in IL-6 levels for both groups. The decrease in both groups could have occurred due to the administration of antipsychotic therapy in both groups which caused a decrease in proinflammatory cytokines, namely IL-6 levels after administration for six weeks, but the decrease in the treatment group was greater than in the control group. This is in line with previous studies where inflammation is thought to have a relationship with the structure and composition of gut microbes. Strains of the genera Lactobacillus and Bifidobacterium are commonly referred to as lactic acid bacteria. Lactic acid bacteria are considered nonpathogenic and are believed to be beneficial to human health. The so-called probiotic effects of lactic acid bacteria can include stimulation of the immune system. Past studies suggest that the initial production of IL-6 showed little variability in repeated experiments and the amount of IL-6 released after stimulation with strains of lactic acid bacteria varied. Most bacteria showed a tendency to be better inducers of IL-6 than the pre-existing bacteria [19]. Biagi et al. [20] found that an increase in the pro-inflammatory cytokine IL-6 was correlated with enriched microbes and a decrease in some butyrate-producing bacteria. The findings of previous investigators support that changes in the gut microbiota profile may play a role in inflammation or be influenced by systemic inflammatory status. Past studies also hypothesized that to achieve intestinal microbiota remodeling, a balance between inflammatory and anti-inflammatory processes must be achieved [21].
Several probiotic strains have been shown to induce in vitro release of proinflammatory cytokines, tumor necrosis factor, and IL-6 which reflect stimulation of nonspecific immunity [7]. Cytokines alter the concentration of several neurotransmitters that regulate communication in the brain including serotonin, dopamine and glutamate. By reducing the total amount of pro-inflammatory cytokines, either directly or by increasing anti-inflammatory cytokines, probiotics that have psychobiotic properties can reduce the cytokines that have access to the central nervous system and may also reverse inflammation that induces the permeability of the blood-brain barrier [22].
For clinical symptoms as measured by the total PANSS value based on the analysis of the treatment group, the total PANSS showed a significant difference (p=0.001) between the initial week, 2nd week, 4th week, and 6th week. This study also obtained a significant improvement in clinical symptoms in the treatment group with moderate improvement (29%±6%) and minimal improvement (15%±9%) in the control group. The decrease in the PANSS value from week to week describes the improvement in clinical symptoms of patients with schizophrenia after receiving therapeutic doses of antipsychotics and probiotic capsules compared to the group that did not receive probiotic capsules. In the initial week of PANSS for the treatment group, the conditions were more varied than in the control group. But, after giving antipsychotics and adjuvant probiotic therapy in the 2nd week, 4th week, and 6th week, the opposite happened so that the decrease in PANSS values was more consistent and better in the group treatment compared to the control group. It can also be seen that adjuvant probiotic therapy improves after at least 2 weeks of administration in subjects receiving the additional therapy. Other studies stated that when conditions are stressed, the hypothalamic-pituitary-adrenal axis is activated and causes the adrenal glands to secrete cortisol, in which probiotics can alter this dysfunction and help reduce stress. Probiotics act as anti-inflammatory agents when inflammation occurs as a marker that sends impulses to the brain via the vagus nerve causing stress but probiotics can minimize the inflammation thereby increasing immune function [23]. Theoretically, various bacteria are capable of producing and secreting neurochemicals that are used for calming. Certain strains of Lactobacillus and Bifidobacterium secrete gamma-aminobutyric acid. The main role of inhibitory neurotransmitters in the brain that regulates many logical and psychological processes with dysfunction in systems involved in anxiety, depression, and even anxiety [24]. Before treatment with probiotics, the flora pattern was similar to that of Enterococcus being the main bacteria which accounted for about 60% of the flora. However, 30 days after the start of treatment, Enterococcus peaks were no longer visible and Clostridium, Ruminococcus, Eubacterium, Catenibacterium, and Bacteroides were detected showing a clear change in the flora pattern. In line with this change, the PANSS score improved slightly. It appears that changes in gut flora improve cytokine balance leading to immune system-mediated beneficial effects on the nervous system. Changes in fecal flora and reduction in negative symptoms noted after treatment with probiotics are suggested to have clinical significance for immunomodulation in patients with schizophrenia [25].
The results of the correlation between PANSS and IL-6 levels in this study showed the dynamics of changes occurred in a better direction where the decrease in PANSS was accompanied by a decrease in IL-6 levels and changes occurred after the 2nd week of administration. Furthermore, there was a weak correlation in each measurement with varying correlation directions, namely in the positive and negative directions. The improvement in PANSS was accompanied by an improvement in IL-6 levels with a decrease in these levels after examination, where the two variables had a unidirectional relationship or a positive correlation. Therefore, the administration of probiotics as adjuvant therapy in patients with schizophrenia can be given at least two weeks before it will have an effect on improving clinical symptoms as measured by PANSS and decreasing IL-6 levels. Previous studies have demonstrated increased serum levels of proinflammatory cytokines in patients with schizophrenia compared with controls and a correlation between levels of inflammatory markers and severity of clinical symptoms. Showing an increased inflammatory response and a shift in the ratio to normal has been associated with a positive response to treatment [4]. The use of probiotics in patients with schizophrenia previously also found a correlation between proinflammatory cytokines and clinical symptoms. The duration of the intervention can also affect the results of the study. However, evidence suggesting a minimal duration of intervention showing a significant effect of probiotic treatment is also very scarce [23]. In one study, intake of the same strain of Bifidobacterium breve A1 significantly reduced the anxiety and depression scale and hospital anxiety and the PANSS at four weeks of administration. The probiotics Ligilactobacillus salivarius (LS 01) and Ligilactobacillus acidophilus (LA 02) were able to significantly decrease levels of proinflammatory cytokines and reactive oxygen species and increase anti-inflammatory cytokines in peripheral blood mononuclear cells from healthy patients and controls [26].
Lastly, it is worth noting that given that most mechanistic studies were conducted using animal models, it is also important to highlight that there are still existing gaps in knowledge regarding the interaction between the microbiome and the host in vivo and the pathway of its metabolites and how their metabolites influence the microenvironment. A study that analyzed colony microbiota against the HPA axis and stress response in mice found that germfree (GF) mice experienced increased adrenocorticotrophic hormone and corticosterone associated with the stress response, and decreased brain-derived neurotrophic factor in the cortex and hippocampus. After receiving the Bifidobacterium infantis microbiome, GF mice underwent a reconstitution of the HPA axis of stress response [27].
In conclusion, the results showed a decrease in the PANSS value which describes the improvement in clinical symptoms of the schizophrenic group after receiving therapeutic doses of antipsychotics and probiotic capsules or the treatment group as well as the schizophrenia group receiving therapeutic doses of antipsychotics and placebo capsules or the control group.
Improvements in clinical symptoms and decreased levels of IL-6 in the group of patients with schizophrenia who received risperidone with probiotic adjuvant therapy were better than in the group of patients with schizophrenia who received risperidone without probiotic adjuvant therapy. The administration of probiotics as adjuvant therapy gave an improvement effect after two weeks of administration in the treatment group.
For the limitations of this study, although probiotic strains can affect research results, there has been no research confirming probiotic strains that are very effective for improving long-term psychological conditions. The single-use of risperidone was not completely found in the treatment group subjects, where there were still those who received additional therapy for sedatives, namely clozapine 25 mg so that the equivalent dose still varied for the treatment group. This study did not control the use of clozapine 25 mg as this was the treatment protocol used at Dadi Psychiatric Hospital. In addition, the use of clozapine as a sedative is also a therapeutic protocol in schizophrenic patients with sleep disorders.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2022.0064.
Comparison of improvement in clinical symptoms based on the difference between baseline and 6th week PANSS in the control group and the treatment group
Correlation between the difference in the value of IL-6 and the difference in total PANSS
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Edy Husnul Mujahid, Erlyn Limoa, Saidah Syamsuddin. Data curation: Burhanuddin Bahar, Rinvil Renaldi. Formal analysis: Burhanuddin Bahar, Rinvil Renaldi. Investigation: Aminuddin Aminuddin, Sonny T. Lisal. Methodology: Edy Husnul Mujahid, Erlyn Limoa, Saidah Syamsuddin. Validation: Aminuddin Aminuddin, Sonny T. Lisal. Writing—original draft: Edy Husnul Mujahid, Erlyn Limoa, Saidah Syamsuddin. Writing—review & editing: Edy Husnul Mujahid, Erlyn Limoa, Saidah Syamsuddin.
Funding Statement
None
Acknowledgements
Thank you to the Committee for the Indonesia Medical Innovation Research Award in Hygiene 2021, the IDI Research Institute and PT. Unilever Indonesia, Tbk for the support so that this research can be carried out.