Pain and Depression: A Neurobiological Perspective of Their Relationship
Article information
Abstract
Remarkable progresses have been achieved regarding the understanding of the neurobiological bases of pain and depression. The principal role of neurotransmitters, neuromodulators, and neurohormones has been proposed in the development of pain and depression. With the progression of molecular biology, an intricate interaction among biological factors accountable to the development and management of pain and depression has been also shown in a numerous preclinical and clinical researches. This mini-review will briefly describe the current issues and future research direction for better understanding of the relationship between pain and depression.
INTRODUCTION
Depression and pain commonly occur together and both can have detrimental effects on patient health, personal and social function, and overall quality of life. Pain is a controversial syndrome and its clear phenomenology, aetiology, pathogenesis and treatment remain a matter of controversy.1 Numerous studies indicate that patients with pain are at increased risk for a lifetime history of depression, as compared to the normal population.2
According to the World Health Organisation study in primary care settings across the world, approximately one-fifth of all primary care patients suffer from persistent debilitating pain, and they are four times more likely to have co-morbid anxiety or depression than pain-free primary care patients.3
Similarly, depressed patients are also associated with more pain complaints and greater impairment in functional capacity. In addition, the risk of depression is greater when the pain is more diffuse, as indicated by the number of painful sites, and has a greater effect on the quality of life.4,5
In addition, serotonin and norepinephrine were principally investigated since these neurotransmitters are located in the main pain pathway as well as preclinical and clinical evidence have suggested a significant role of such neurotransmitters regarding the development and treatment of pain.6 In fact, a number of psychotropic agents such as selective serotonin reuptake inhibitors (SSRIs), noradrenergic and specific serotonin receptor antagonist (NaSSa), serotonin-norepinephrine reuptake inhibitors (SNRIs) have been actively tried for the treatment of pain disorders or painful somatic symptoms regardless of patients' diagnosis, all of which are involved in serotonin and norepinephrine neurotransmission.6
This brief perspective will provide a summary of intricate relationship between pain and depression as a further understanding on the role of pain in relation with the pathophysiology, clinical manifestation, and proper management of depression.
COMMON UNDERSTANDING ON ETIO-PATHOLOGIC MECHANISM
Overall findings
The precise etio-pathological mechanism of pain remains still unclear. A number of clinical and biological factors have been demonstrated to be involved in the development of pain. For instance, altered neuromodulation,7 abnormalities of the immune-genetic system (i.e., alterations in pro-inflammatory cytokines8 and their gene expression),9 alteration in melatonin,10 aberration in 3H-imipramine binding sites on platelets,11 the oxidative stress reaction,9 a disturbance of neurotransmitters,12 stress susceptibility,13 alterations of hypothalamus-pituitary-adrenal axis function,14 and cognitive vulnerability15 are known to at least partly play a role in the manifestation of pain. In addition, a number of psychosocial factors (i.e., self esteem, self-efficacy, anger, sexual abuse, personality profile, early childhood abuse) are also crucially involved in the development of pain.16,17,18 Intriguingly all of which clinical and biological factors aforementioned are also common in the development of depression.
Genetic factors
Patients with chronic pain have more first degree relatives with depression compared with the general population.3,19 Significantly higher rates of depression have been found in family members even for patients with chronic pain without a personal history of depression.3,11 According to a previous human genetic study,20 the galanin-2 and mu opioid receptors are plausible candidates for mediating the effects of pain on mood, as they show graphic patterns consistent with a pain-gene interaction, although such specific effects cannot be conclusive due to the modest sample size of the study. In addition, a recent study found that serotonin 1A receptor (5HTR1A) and serotonin 2A receptor gene promoter variations have gender-dependent modulatory effects on depression and physical function in patients with pain. Furthermore, that study demonstrated that pain after lumbar surgery modulates the association between 5HT gene polymorphisms and depression.21 These findings suggest that pain sensitivity, the stress response and mood regulation share common familial-genetic factors and support the hypothesis that depression and pain are genetically related.
Pain pathways and biochemical findings
The CNS pathway responsible for inhibition of pain sensation includes projections from brainstem nuclei to the spinal cord dorsal horn via the dorsolateral funiculus (DLF). More specifically, DLF fibers are comprised of serotonergic projections from the raphe nuclei, dopaminergic projections from the ventral tegmental area (VTA), and noradrenergic projections from the locus coeruleus.22,23 These descending fibers suppress pain transmission at the nociceptive spinal cord neurons presumably by hyperpolarizing afferent sensory neurons using endogenous opioids, or serotonin and norepinephrine as principal inhibitory mediators.22,23
Activity induced in the nociceptors and nociceptive pain pathways is not pain, but is nociception, and pain perception is formed in the central nervous system as a very interactive phenomenon through central and peripheral systems. The pathophysiology of acute pain is fairly clear, but chronic pain is still a great enigma, and includes a number of neurotransmitters (GABA, glutamate, norepinephrine, serotonin, neurokinin 1, nitric oxide, substance P, glycine and opioids).24 It has been proposed that central and peripheral pain mechanisms may be altered in patients with pain, resulting in amplified pain signals.25 Current theories assume that continuous stimulation of the pain pathways (C-fibres) leads to overactivation of N-methyl-D-aspartate receptors in the dorsal horn of the spinal cord.26 In the first human study investigating that repetitive stimulation enhances a bidirectional mechanism of secondary hyperalgesia due to viscerosomatic facilitation in patients with irritable bowel syndrome (IBS),27 the IBS patients were tested by repetitive stimulation of rectal and somatic tissues to induce central sensitization as well as an NMDA receptor antagonist (dextromethorphan) versus placebo. According to the results, enhanced pain sensitivity was nearly completely prevented by administration of dextromethorphan, suggesting that pharmacological manipulation of NMDA receptor mechanisms is essentially involved in modulation of central sensitization for processing of pain.
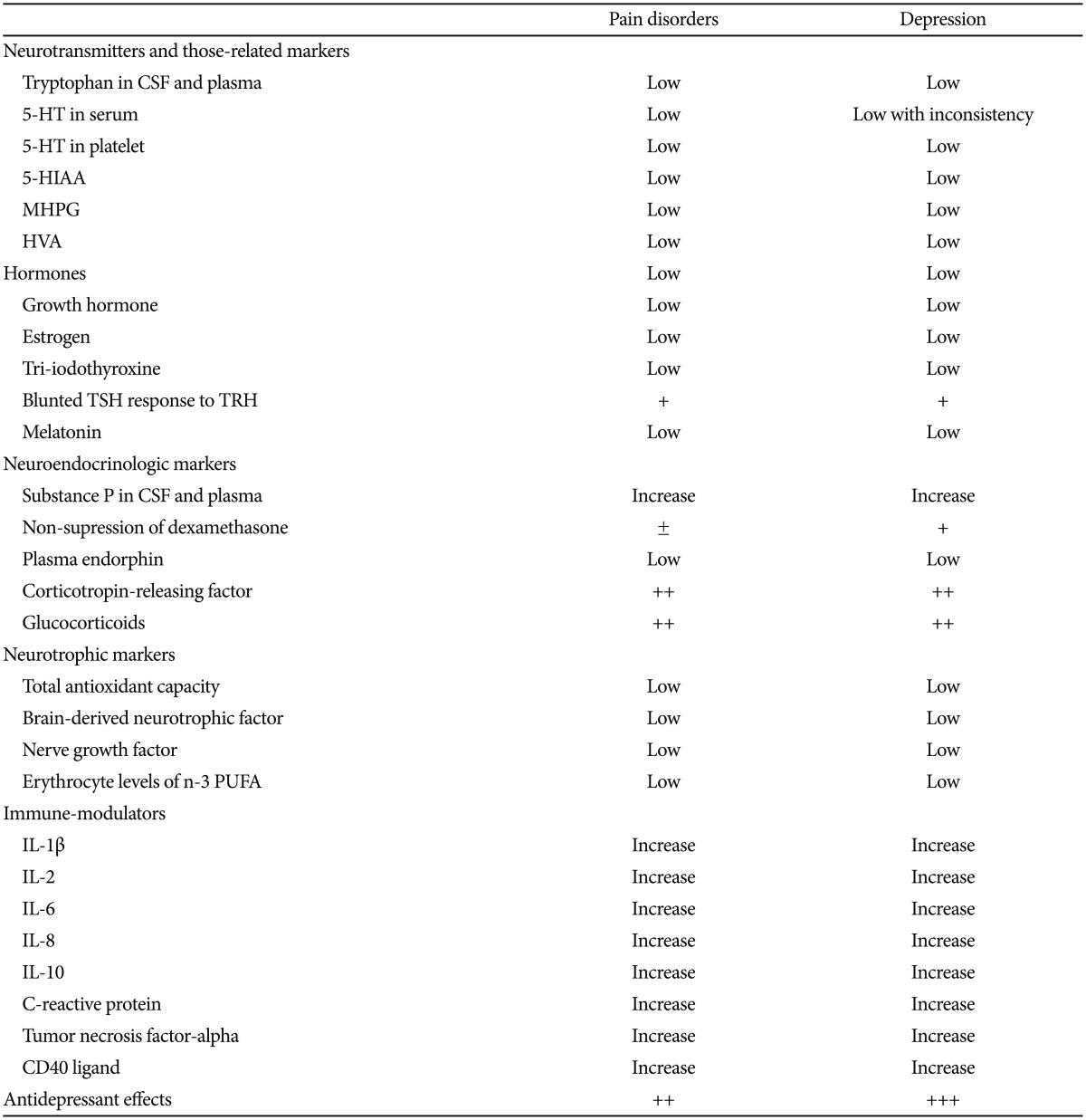
Increased secretion of excitatory neuromodulators, such as substance P, and psychological factors further escalate such pain perception. The resulting state is referred to as "central sensitisation" wherein intense pain is produced from innocuous stimuli.28 For example, patients with pain show reduced blood flow in the caudate and thalamic areas involved in the central processing of noxious signals29 and elevated concentrations of cerebrospinal fluid substance P30 and brain-derived neurotrophic factor.31 In a recent study,32 enhanced central pain sensitivity at both ends of the spinal neuroaxis was found in patients with fibromyalgia and therefore widespread central sensitisation. These findings are commonly observed in patients with depression as well.33,34 Table 1 represents the potential similarities in biological markers between pain disorders and depression.
The principal biochemical basis for pain and depression has focused on the neurotransmitters serotonin and norepinephrine. Both the serotonin and norepinephrine pathways in the brain and their associated symptoms have been determined. As described before, both pathways originate in the brain stem nuclei and project to multiple brain regions such as somatosensory cortex, intralaminar nuclei and ventral posterior nuclei of the thalamis.35 In the context of pain regulation, peripheral pathways descend projections from the brain stem to the spinal cord. The serotonin and norepinephrine descending neurons project from the brain stem into the spinal cord's dorsal horn, where a set of biochemical cascades takes place involving other types of neurotransmitters for controlling pain.35 The Figure 1 represents schematic illustration of the pain pathway.

The physiological pathway of pain. FC: frontal cortex, PSC: primary sensory cortex, HT: hypothalamus,CC: cingulated cortex, AMD: amygdala, MTN: medial thalamic nuclei, LTN: lateral thalamic nuclei, FNE: free nerve ending. The whole process of perception of pain is following: Transduction → Transmission (central sensitization) → Modulation → Perception.
Disturbed cognition
Many cognitive variables have been shown to mediate the pain-depression association, such as pessimism, perceived locus of control, fear avoidance beliefs, self-efficacy, and perceived social support.36,37 Perhaps the best studied and potentially most relevant to the pain-depression relationship is catastrophizing that is literally to view or present a situation as considerably worse than it actually is. Specifically, in psychological researches, it refers to cognitive and emotional processes encompassing magnification of pain-related stimuli, feelings of helplessness, and a generally pessimistic orientation. The term was initially introduced by Albert Ellis and subsequently adapted by Aaron Beck to describe a mal-adaptive cognitive style employed by patients with anxiety and depressive disorders.38 Hence, the catastrophizing is currently considered a cognitive error where one assumes the worst possible outcome will occur.39 It has been associated with pain and depression separately, and they co-occur as well.37 Depression and catastrophizing has been found to be associated with higher pain severity and functional incapacity, conversely, researches also propose that those who catastrophize their pain tend to feel helpless, dreadful, and even worthless.37 Recent research supporting such viewpoint studied 669 older adults with chronic pain and found that catastrophization mediated the relationship between pain intensity and depression.40
Overlapping clinical symptoms
Patients with pain and depression have important similarities, as reflected in elevated levels of depressed mood,41 alexithymia,42 anger,43 decreased energy and fatigue,44 cognitive defect,45 increased vulnerability to stress,46 heart rate variability47 and sleep disturbance (decreased REM latency).48 Furthermore, the severity of pain is highly associated with some depression-related symptoms and impairments such as depressed mood, fatigue, functional disability, suicidal ideation, poor daily health status and more somatic symptoms.45,49,50
Effects of antidepressants and cognitive therapy
Despite no evidence for the superiority of one class of antidepressants in the treatment of pain, contemporary antidepressants involving serotonin and norepinephrine activities such as venlafaxine, duloxetine and milnacipran have consistently shown the promising effect for controlling pain among variable psychotropic agents.
However, recently, in a number of clinical trials with dopaminergic agents have also been conducted and their beneficial effects for controlling pain51 and depression19 have been demonstrated. A recent study investigated whether or not pain-related behavioural depression is mediated by activation of endogenous κ-opioid systems and subsequent depression of mesolimbic dopamine release.52 That study found a crucial role of dopamine neurotransmission in terms of pain-related depression of behaviour, pain-related depression of mesolimbic dopamine release and role of endogenous dynorphin/κ-opioid receptor systems: 1) the acid noxious stimulus also depressed extracellular levels of the neurotransmitter dopamine in nucleus accumbens; supporting the fact that depression of mesolimbic dopamine release may contribute to negative affective dimensions of pain; 2) acid-induced depression of dopamine release was blocked by both NSAID and opioid analgesics, indicating the potential relationship of opioid with affective dimensions of pain, and 3) alteration of mesocorticolimbic prodynorphin expression by noxious stimulus.52 In addition, intriguingly, milnacipran does not significantly modulate the activity of segmental spinal nociceptive neurons or influence the diffuse noxious inhibitory control activity or the documented thermoalgic allodynia, but the supraspinal analgesic effect might be involved in the mechanism of milnacipran for controlling pain.53 For instance, dopaminergic agents for pain conditions like fibromyalgia and neuropathic pain, showed varied results. In a study with pramipexole, a dopamine receptor agonist, for the treatment of fibromyalgia, the primary outcome was improvement in the pain score [10-cm visual analog scale (VAS)] at 14 weeks.51 Compared with the placebo group, patients-treated with pramipexole experienced gradual and more significant improvement in measures of pain. At the endpoint, the VAS pain score decreased 36% in the pramipexole arm and 9% in the placebo arm, indicating a huge magnitude of difference of 27%. Forty-two percent of patients receiving pramipexole and 14% of those receiving placebo achieved ≥50% decrease in pain. This study has clearly established the efficacy and safety of pramipexole in a subset of patients with fibromyalgia.51 Bupropion, a representative antidepressant acting on dopamine modulation, has been also tested in the management of pain.54 In an outpatient-based (n=42), randomized, double-blind, placebo-controlled, crossover study consisted of two phases (12 weeks), bupropion significantly reduced the mean average pain score by 1.7 points to 4.0 (p<0.001), while placebo treatment failed to do so. Such effect started to be significant at week 2 and remained throughout weeks 3 through 6 (p<0.001). A significant decrease in interference of pain on quality of life was also observed, while it was not shown in placebo treatment.
In this context, it appears reasonable that all three major neurotransmitters are related to the pathophysiology of depression/pain and, as such, are relevant targets for pharmacological intervention. Triple reuptake inhibitors under development, which enhance neurotransmission of all three systems, should be a potential next-generation therapeutic agent to provide more reliable efficacy and a quicker therapeutic effect.55 Hence, enhancing the current understanding of the exact action mechanism of antidepressants on pain pathways should also be simultaneously examined to expand our knowledge, thus leading to better pharmacological management and clinical outcomes for such patients.
Psychological interventions took a main position in modern pain management practice and have also been a recommended option of a modern pain treatment service. A number of systematic reviews and meta-analyses have been conducted and provided the evidence of psychological interventions for the management of pain in clinical practice.56,57 Among such psychological interventions, cognitive behavioural therapy (CBT) with a focus on cognitive coping strategies and behavioural rehearsal has demonstrated the strongest evidence. However, currently available most evidence is for treatments of adult pain but few for adolescent and older patients with chronic pain.58 According to such well-researched systematic reviews, beneficial effects without any harmful effects by using any medications were prominently evident with CBT.56,59 It has been proven that substantial improvement in pain, mood, and functional incapacity which were not able to be achieved by any other form of treatment, was also remarkable. However, the overall effect sizes of CBT for adults, across all trials, were modest.56
DISCUSSION
Despite the fact that pain and depression potentiate each other clinically, and an increasing number of neuroimaging studies show that physical pain and depression involve the insular cortex,60 a recent functional imaging study61 clearly suggested that they may involve different cortical pain areas. According to that study, patients with depression show less activation of the operculum and the secondary somatosensory cortex, as compared to that in patients with pain, whereas patients with pain demonstrate increased activation in the anterior insula and parietal operculum. In addition, in all participants sensitised to pain, there was an association with higher activation levels in the thalamus, amygdala, mid-cingulate cortex and sensory and motor areas; however, depressed patients showed significantly less activation in midbrain and brainstem areas. Hence, newer imaging techniques, possibly proving the existence of different neuronal correlates noninvasively between pain and depression in terms of brain metabolism and hemodynamics during acute and chronic phases, would also be helpful in understanding both conditions.
Emerging evidence suggests that dopamine may be another crucial factor associated with symptoms of pain and depression, whereas previous pain and depression studies have focused mainly on serotonin and norepinephrine. In fact, some anecdotal reports have proposed that the VTA is related to the processing of nociceptive information as well as control of motor function. In fact, in a recent rodent study,62 VTA lesions enhanced the occurrence of self-injury behaviour, while activation of the VTA by electrical stimulation after a nociceptive stimulus, but not before, facilitated the analgesic process, indicating that the VTA should play a key role in the processing and modulation of persistent pain information. Fian and Smith63 have also proposed that the homeostatic regulation of tonic and phasic dopamine may contribute to both pain and depression. Hence, attention to the role of dopamine in pain and depression would help clinicians understand both clinical conditions more fully.
Although increasing evidence indicates that the relationship between both clinical conditions might be bidirectional,64 the question remains whether the specific neurobiological changes cause either pain or depression or are merely a consequence of each condition. Interestingly, some researchers proposed the concept of chronic pain as a psychological disorder since there is some support to view chronic pain as the prime expression of a muted depressive state;65 however, it has been remained a vague entity due to a lack of continuous supporting data for this notion. Integrative and comprehensive approaches for revealing the association between pain and depression would be more straightforward and reasonable based on currently available findings.
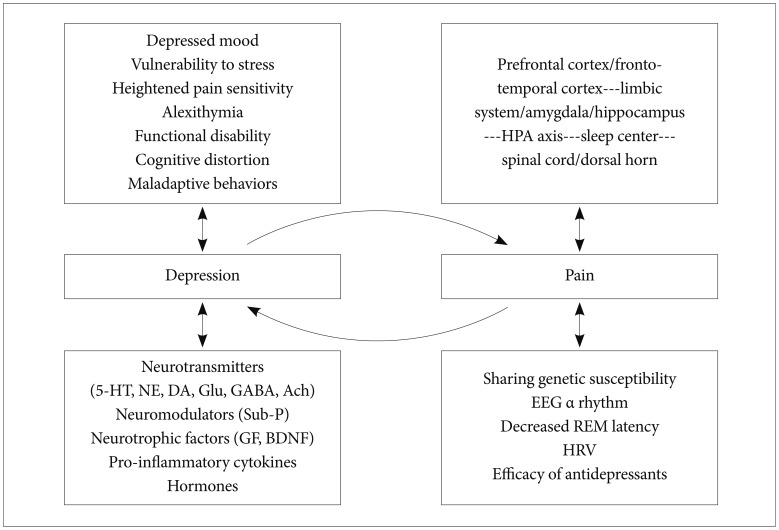
Depression and pain share clinical manifestations, neurobiological pathways, neurotransmitters, hormones, stressors, neurotrophic factors and pro-inflammatory cytokines, which have implications for the development and treatment of both simultaneously. The final common pathway in which chronic pain and depression may be ultimately associated should be when both arise out of a common underlying process.
Another huge interesting point is that we need to consider the relationship between suicide and pain since the suicide rate is higher in patients with depression as well as with pain, indicating that pain could be a potentially common mediator or risk factor for both clinical conditions. In fact, clinical data consistently suggest that patients with medico-surgical conditions including malignancies and cardiovascular diseases causing serious disability of patients or simple but chronic headaches are at an increased risk of committing suicide.66,67,68,69 For instance, according to large-scale studies, patients with migraine are from 2.2 to 4.0 times more likely to have depression70 and as well as more likely to have suicide mortality (odds ratio=1.68).69 In addition, patients with migraine have been found to be associated with more increased risk of developing suicidal ideation and committing suicide attempts when they are comorbid with depression.70 Hence, it should be also intriguing for clinicians to investigate how pain and depression are interactive and implicated in the development and prevention of suicide.
Next researches should also try to clearly establish the relationship between acute and chronic pain with depression. In fact, acute and chronic pain are different clinical entities. Acute pain is usually provoked by a specific disease or injury and is self-limited, while chronic pain may be considered as a disease state and also arise from psychological states.71,72 Chronic pain has been proposed to be the consequence of persistent impulses in somatosensory pathways with related CNS changes (i.e., altered local brain chemistry and functional reorganization due to central plasticity).73 In addition, the management and treatment of acute and chronic pain patients are also substantially different due to its basic differences in clinical course and symptoms. However, there has been a paucity of data concerning a differential relationship between acute and chronic pain and depression till today. Hence this clinical issue warrants well-designed future studies.
Contemporary findings suggest that understanding the brain is a critical and indispensable component of pain and depression research involved in explaining the ways in which biological, psychological, behavioural and social factors influence both pain and depression, and offers a way to the optimal therapeutic approach (Figure 2).24 Psychosocial and behavioural factors play a significant role in the experience, maintenance, and exacerbation of pain.37 CBT alone or within the context of an interdisciplinary pain rehabilitation program has the greatest empirical evidence for success.37 As none of the most commonly prescribed treatment regimens are sufficient to eliminate pain, a more realistic approach will likely combine pharmacological, physical, and psychological components tailored to each patient's needs.59
Therefore, a bio-psycho-social model that integrates aetiology, evaluation and management of depression and pain concurrently is mandatory for better care of such patients. We must promote further theoretical, methodological and technical advances to clarify the precise relationship between these conditions, which may also lead to more effective biological and psychological therapies for such patients.
Acknowledgments
This study was partially funded by the Ministry of Health and Welfare, Korea (HI12C0003).