Atypical Antipsychotics Mediate Dynamics of Intrinsic Brain Activity in Early-Stage Schizophrenia? A Preliminary Study
Article information
Abstract
Objective
Abnormalities of static brain activity have been reported in schizophrenia, but it remains to be clarified the temporal variability of intrinsic brain activities in schizophrenia and how atypical antipsychotics affect it.
Methods
We employed a resting-state functional magnetic resonance imaging (rs-fMRI) and a sliding-window analysis of dynamic amplitude of low-frequency fluctuation (dALFF) to evaluate the dynamic brain activities in schizophrenia (SZ) patients before and after 8-week antipsychotic treatment. Twenty-six schizophrenia individuals and 26 matched healthy controls (HC) were included in this study.
Results
Compared with HC, SZ showed stronger dALFF in the right inferior temporal gyrus (ITG.R) at baseline. After medication, the SZ group exhibited reduced dALFF in the right middle occipital gyrus (MOG.R) and increased dALFF in the left superior frontal gyrus (SFG.L), right middle frontal gyrus (MFG.R), and right inferior parietal lobule (IPL.R). Dynamic ALFF in IPL.R was found to significant negative correlate with the Scale for the Assessment of Negative Symptoms (SANS) scores at baseline.
Conclusion
Our results showed dynamic intrinsic brain activities altered in schizophrenia after short term antipsychotic treatment. The findings of this study support and expand the application of dALFF method in the study of the pathological mechanism in psychosis in the future.
INTRODUCTION
Schizophrenia is a severe, chronic psychiatric disease with a high disability, characterized by core symptoms including disorganized thoughts, delusions, and hallucinations [1,2]. It places great burden not only to the patients and their families, but to the whole society as well [3-5]. However, the pathological mechanisms leading to schizophrenia still remain unknown to a large extent due to the differences in clinical manifestations and treatment responses.
Resting state fMRI (rs-fMRI) is an ideal way to characterize spontaneous brain activity free from external stimulation and to identify brain networks with common functional patterns [6]. It could detect abnormal intrinsic brain activities in mental illness effectively without the need for complex cognitive tasks and experimental design [7-9]. As a reliable imaging marker, amplitude of low-frequency fluctuations (ALFF) has been widely applied in the measurement of regional spontaneous neuron disturbances in schizophrenia in recent years [10-13]. Reduced ALFF may represent a functional impairment related with diseases, while increased ALFF may demonstrate a compensatory for maintaining normal cognition [14]. Although many imaging studies have found ALFF alterations in schizophrenia, the results are mixed and inconsistent. Some studies reported only increased ALFF and some reported only decreased ALFF in schizophrenia patients compared with healthy subjects. Liu et al. [11] observed that increased ALFF including the middle temporal, orbito-frontal, inferior occipital and fronto-insular gyrus in patients compared with healthy controls. Guo et al. [13] reported that patients with schizophrenia exhibited decreased ALFF in the right Crus I. But most studies found both decreased and increased ALFF in patients. Li et al. [15] found that ALFF in the bilateral cerebellum posterior lobe was significantly decreased in patients, while ALFF in the right fusiform gyrus and the bilateral putamen was significantly increased. Zhou et al. [16] reported that schizophrenia patients showed decreased ALFF in the sensorimotor area, visual cortex, and frontoparietal pathway, but increased ALFF in the precuneus and middle cingulate gyrus when compared with the HC. These mixed findings may be partly attributed to the oversimplification of the data due to the “static” analysis of brain activity. Most of the studies relied on the assumption of constant brain activity over the length of the scan and did not investigate the time-varying properties of the intrinsic brain activities in patients. We used to consider the brain to be in an stable state at rest so that the presence and potential of temporal variability are commonly ignored among the investigations of intrinsic brain activities based on rs-fMRI [17], but now we have learned that the brain is a highly dynamic nervous system with rapidly changing neural activity and keep dynamic balance constantly [17-19]. More recently, researches are focused on dynamic functional connectivity (dFC) properties which reflect the evolving brain network allocation and may be related to changes in neural communication patterns that serve specific brain functions [18,20]. The intrinsic brain activity is regarded as a reflection of various and varied mental processes [21], which may also have highly time-varying properties [22]. Fu et al. [18] explored the time-varying patterns in brain activity. They found the ALFF of brain regions fluctuated highly at rest and this dynamic pattern was altered in schizophrenia. Though many studies have found abnormal dFC in schizophrenia compared with healthy subjects [23-26], researches on how dynamic ALFF (dALFF) changes in patients and how antipsychotics affect dynamics of intrinsic brain activity remain few. Comprehending the time varying brain activity enables us to better understand the underlying mechanisms of brain dynamics. In addition, how antipsychotics affect them deserve further investigation.
To understand the temporal variability of intrinsic brain activities in schizophrenia and the short-term effects of atypical antipsychotics on it, we employed dALFF analysis on rs-fMRI data in first-episode, drug-naïve schizophrenia patients at baseline and after 8-week antipsychotic treatment. We hypothesised that aberrant dALFF could be observed in the patients and dynamic brain activities could be altered with the improvement of the symptoms after the medication.
METHODS
Participants
A total of 52 individuals were participated in this study, including 26 schizophrenia patients (SZ) and 26 healthy controls (HC). The SZ group was recruited from the outpatient and inpatient departments of Shanghai Mental Health Center (SMHC), and the HC group was recruited through advertising. All the patients were suffering from schizophrenia or schizophreniform disorder using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, (DSM-IV) criteria and met the inclusion criteria of 18–40 years of age, at the first acute episode with course of illness <5 years, free of antipsychotics, and total score on the Positive and Negative Syndrome Scale (PANSS) ≥60. 27,28 Patients who diagnosed with schizophreniform disorder were subsequently diagnosed with schizophrenia after 6 months of duration observation. The exclusion criteria were 1) major neurological diseases; 2) history of head trauma; 3) history of drug or alcohol abuse or dependence; 4) electroconvulsive therapy within 6 months; 5) women in pregnancy or breastfeeding; 6) in unstable state such as aggressive; 7) diagnosis of any other mental diseases.
After baseline scan, all the patients received atypical antipsychotics in a naturalistic treatment. The type and dose of medication was up to their clinicians. Symptoms were evaluated using the Positive and Negative Syndrome Scale (PANSS) [28], the Assessment of Negative Symptoms (SANS) [29], and the Assessment of Positive Symptoms (SAPS) by the same psychiatrist at baseline and follow up [30]. Twenty-six subjects matched on age, gender and handedness were recruited as healthy controls and were assessed using M.I.N.I. plus v 5.0 [31]. The exclusion criteria of the HC group were consistent with those of the SZ group. A current or past history of any sort of psychiatric or neurological illness or a family history of psychosis was an additional exclusion criterion. The SZ group was scanned at baseline (SZt1) and after 8-week treatment (SZt2), while the HC group was scanned only at baseline (HCt).
This study was approved by the Institutional Review Board of Shanghai Mental Health Center (2017-36R) and conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from each subject.
Imaging data acquisition
Imaging data was acquired using a Siemens Verio 3.0 T MRI scanner (Siemens Magnetom Trio, Erlangen, Germany) at SMHC. During the scanning, all the participants were asked to stay awake and keep eyes closed [32]. Structure images were obtained with the parameters as follows: repetition time (TR)= 2,300 ms, echo time (TE)=2.98 ms, 240×256 matrix, flip angle 9°, field of view=256 mm, voxel size=1×1×1 mm3 , slice thickness=1 mm, gap=0 mm, and 196 slices. A gradient-echo (GRE) echo-planar imaging (EPI) sequence was used to acquire the functional images with the following parameters: TR=2,000 ms, TE=35 ms, matrix 64×64, flip angle 90°, field of view=256 mm, voxel size= 3×3×3 mm3 , slice thickness=4 mm, gap=0 mm, and 33 slices.
Data preprocessing
Data preprocessing was conducted using the SPM12 (The Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, London, UK) (www.fil.ion.ucl.ac.uk/spm) and the DPABI software (Key Laboratory of Behavioral Science and Magnetic Resonance Imaging Research Center, Institute of Psychology, Chinese Academy of Sciences, Beijing, China) (http://rfmri.org/dpabi). First, we removed the first ten volumes. For each subject, the mean frame displacement (FD) was calculated based on a previously published formula. 33 The head motions of all subjects were less than the 2 SD of the FD. In addition, we compared the head motion between the patient group (mean FD=0.07, SE=0.014) and control group (mean FD=0.08, SE=0.016). We found that the head motion showed no difference between the two groups (T=0.193, p=0.848). After slice timing and realignment, the remaining functional images were normalized into the Montreal Neurologic Institute (MNI) template and re-sampled at a resolution of 3×3×3 mm3 . After normalization, the linear trend was removed from the time series. Nuisance signals including 24 head-motion parameters and cerebrospinal fluid signals were regressed out by using a multiple linear regression model. We did not regress out the white matter signals because the previous studies found that the white matter fMRI signals also have some pathological information in schizophrenia [8,34-36]. The time series were temporal filtered (0.01–0.10 Hz). Finally, the data spatially smoothed with a 6 mm full-width at half maximum Gaussian kernel.
Dynamic ALFF computation
The dynamic amplitude of low-frequency fluctuation (ALFF) was calculated to characterize the dynamics in brain activity according to a previously published sliding-window method [37]. In brief, the time series were segmented into many windows by using a sliding-window strategy. Previous study reported that a longer window length may overlook the potential temporal dynamics of ALFF and a shorter window length less than 1/fmin may increase the risk of introducing spurious fluctuations [38]. The fmin is the minimum frequency of fMRI signals. Thus, the optimal window length was set at 50 TRs (100s) with a sliding step of five TRs (10s). The full-length of preprocessed time series was 190 TRs (380s), which were divided into 29 windows for each subject. For each sliding window, the ALFF map was calculated, and this could obtain 29 ALFF maps, reflecting the time varying brain activity for each subject. Subsequently, the standard deviation (std) value was further computed across all the 29 ALFF maps to measure the temporal variability in brain activity dynamics. Finally, the std-value of each voxel was divided by the global mean std-value to improve the normality. The normalized std map was defined as the dynamic ALFF map for the subsequent statistical analyses.
Statistical analysis
Demographic information was evaluated between the SZ group and the HC group. The differences in age and education between the two groups was compared by two sample t-test and difference in gender was analyzed by χ2 test.
To determine group differences in the dynamic ALFF at baseline, two sample t-test was performed for comparisons of dynamic ALFF maps between the SZt1 group and HCt group. Age, gender, education, and mean FD were used as covariates. To investigate longitudinal changes of the dynamic ALFF, paired t-test was used to compare differences of dynamic ALFF maps between the SZt1 group and SZt2 group. Multiple comparisons correction was performed using the Gaussian random filed theory (voxel p<0.001; corrected cluster p<0.05). The mean dynamic ALFF value in regions with significant group difference or longitudinal change were extracted. Relationship between the dynamic ALFF at baseline and clinical variables was assessed using Spearman rank correlation analysis. In addition, we calculated the difference values of dynamic ALFF between follow-up and baseline. Spearman rank correlation was performed to further test the association between longitudinal difference of dynamic ALFF and reduction ratio of PANSS, SANS and SAPS.
RESULTS
Demographic and clinical features
No subject was excluded due to excessive head motion and no patient lost to the follow-up visits. Thus, the final sample included 26 patients and 26 healthy controls. All patients received atypical antipsychotics, 15 patients received monotherapy: paliperidone (n=6), risperidone (n=4), olanzapine (n=3), quetiapine (n=1), and aripiprazole (n=1), 11 patients received combined medication (antipsychotic combination): aripiprazole and olanzapine (n=2), risperidone and olanzapine (n=2), aripiprazole and paliperidone (n=2), quetiapine and paliperidone (n=2), ziprasidone and olanzapine (n=2), and risperidone and quetiapine (n=1). The average dosage in olanzapine equivalence was 18.92±4.85 mg/d [39]. Due to the limited sample size we did not perform further analyses to separate drug specific effects.
The demographic and clinical features of the two groups were showed in Table 1. No significant difference was found between the SZ and HC concerning age, gender and handedness; however, the HC had higher levels of education (p=0.002) and as a result, education was controlled for in the subsequent analysis. Table 2 shows changes in symptoms of patients with schizophrenia pre- and post- 8-week antipsychotic treatment.
Temporal variability of dynamic ALFF
Figure 1 shows the group-level dynamic ALFF maps, which were obtained by averaging across all subjects in each group. Consistent with previous studies, these dynamic ALFF maps showed a non-uniform spatial distribution in both HC and SZ groups, either in the baseline or the follow-up. At the baseline, t-test indicated that the SZt1 group showed increased dynamic ALFF in right inferior temporal gyrus (T=4.88, p<0.001), compared with the HCt group (Figure 2). Paired t test showed that compared with the baseline, patients at follow-up exhibited increased dynamic ALFF in the left superior frontal gyrus (T=5.59, p<0.001), the right middle frontal gyrus (T=5.79, p<0.001), and the right inferior parietal lobule (T=5.51, p<0.001) and reduced dynamic ALFF in the right middle occipital gyrus (T=-4.47, p<0.001) (Table 3 and Figure 3).
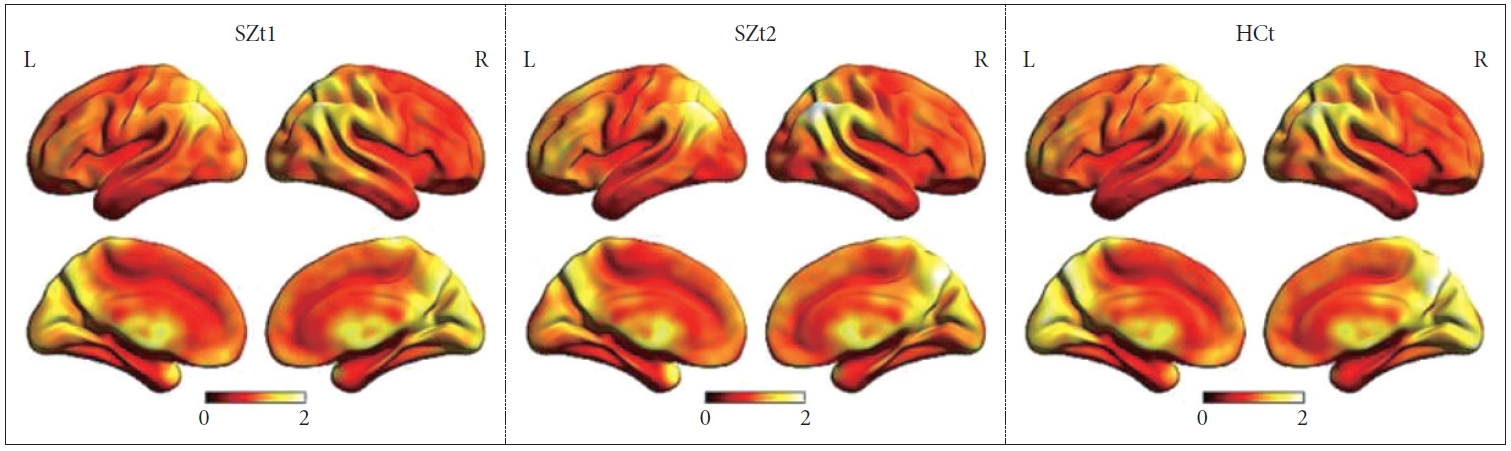
The group-level dynamic ALFF maps obtained by averaging across all subjects in each group. SZt1, the schizophrenia group who was scanned at baseline; SZt2, the schizophrenia group who was scanned after 8-week treatment; HCt, the HC group who was scanned only at baseline; L, left; R, right.
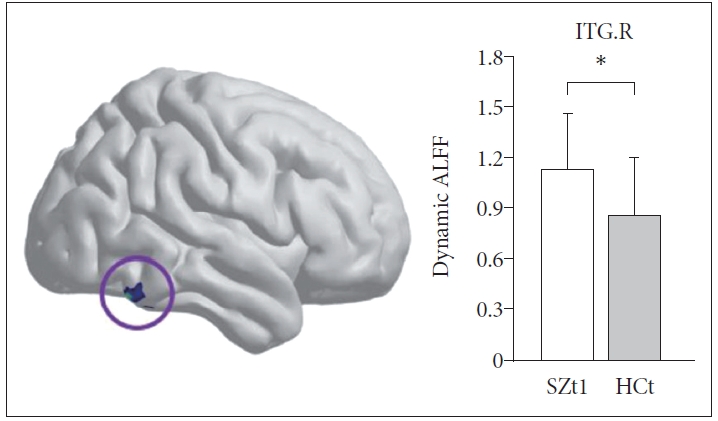
At the baseline, the SZt1 group showed increased dynamic ALFF in right inferior temporal gyrus (T=4.88, p<0.001) compared with the HCt group. *means the mean dynamic ALFF value in this region with significant group difference. ITG.R, the right inferior temporal gyrus.
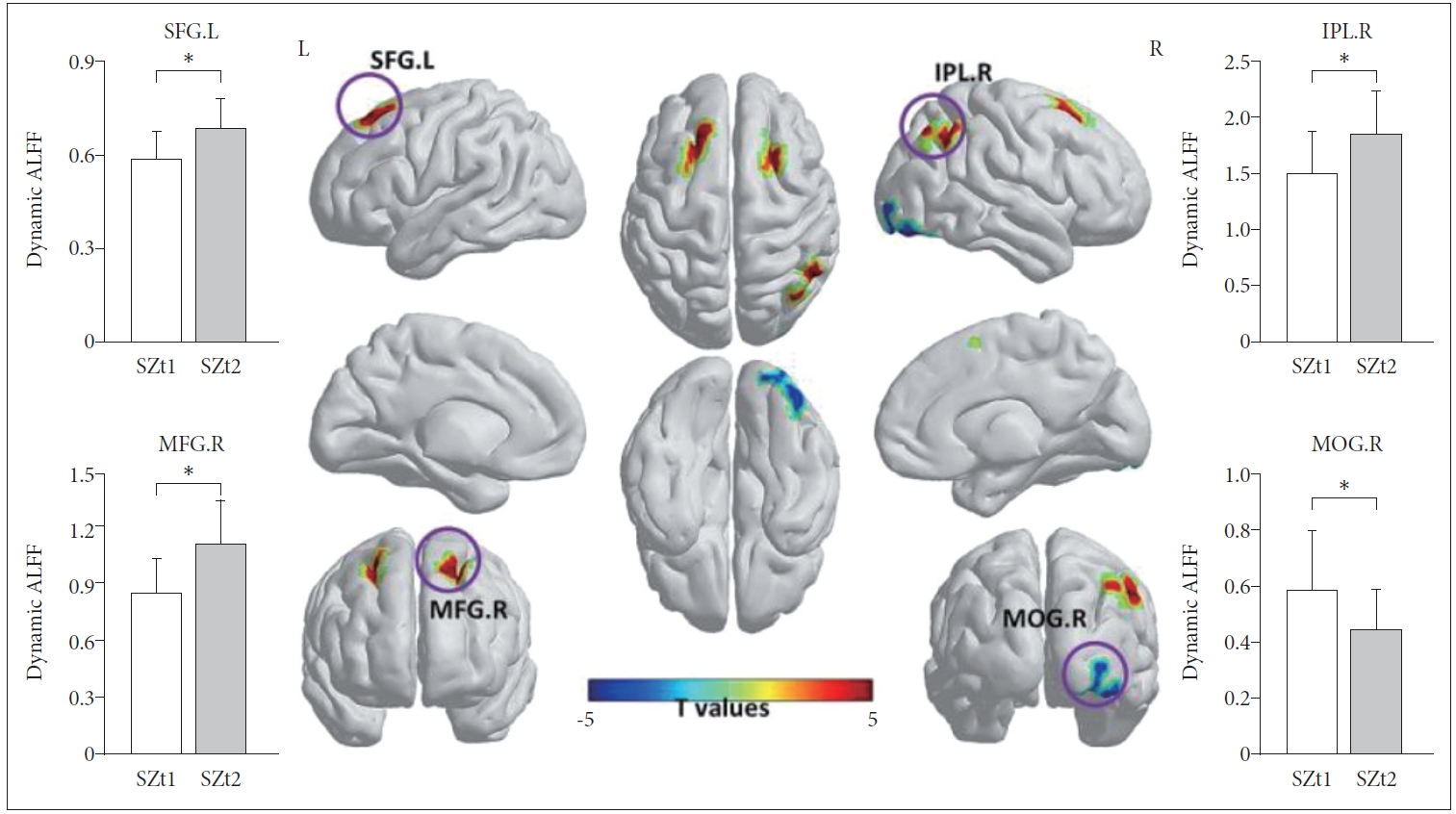
Compared with the baseline (SZt1), patients at follow-up (SZt2) exhibited increased dynamic ALFF in the left superior frontal gyrus (T=5.59, p<0.001), the right middle frontal gyrus (T=5.79, p<0.001), and the right inferior parietal lobule (T=5.51, p<0.001) and reduced dynamic ALFF in the right middle occipital gyrus (T=-4.47, p<0.001). *means the mean dynamic ALFF value in this region with significant group difference. IPL.R, right inferior parietal lobule; MFG.R, right middle frontal gyrus; SFG.L, left superior frontal gyrus; MOG.R, right middle occipital gyrus.
Relationship between dynamic ALFF and clinical variables in schizophrenia patients
At the baseline, we found a significant negative correlation between dynamic ALFF in the right inferior parietal lobule and SANS scores (r=-0.390, p=0.049). However, the correlation was no longer significant at the follow-up. There was no significant association between longitudinal difference of dynamic ALFF and clinical scale reduction ratio.
DISCUSSION
We utilized rs-fMRI to explore brain dynamics in schizophrenia and the effects of treatment with atypical antipsychotics by using a temporal variability of amplitude of low-frequency fluctuations (dALFF). Scans were collected in a longitudinal study design, pre- and post- 8 weeks of antipsychotic treatment. Compared with HC, SZ patients showed increased brain dynamics (more temporal variability of dALFF) in the ITG.R at baseline. After 8 weeks of treatment, we observed both increased and decreased dALFF in different brain regions.
The ITG is responsible for the recognition of objects, faces, and characters, perceiving spatial relations with a higher level of information processing capacity. Zhang et al. [40] found that both increased dynamic fALFF and increased dynamic ReHo were observed in sensory and perception network (including temporal gyrus) in schizophrenia patients. A systematic review including 1,249 schizophrenia patients and 1,179 healthy control subjects displayed increased ALFF in the left inferior temporal gyrus in patients with chronic schizophrenia but not with first-episode, and found that ALFF alterations in the ITG were associated with PANSS score in all patients [14]. Li et al. [37] reported that abnormalities of dALFF in the ITG may increase the risk of mood instability such as impulsiveness, irritability, even suicidal ideation which closely relate to mental disorder patients.
Our results demonstrated that dynamic intrinsic brain activities altered after antipsychotic treatment. In the present study, a significant increased brain dynamic in SFG.L, MGF.R, and IPL.R and a decreased dALFF in MOG.R were observed after medication. Meta-analysis shows that decreased ALFF in the left SFG was found with the most robust data replicable in multiple studies in patients with first-episode schizophrenia [14]. SFG.L and MGF.R are active in the maintenance of cognitive control, while IPL.R are involved in transient cognitive control including performance feedback [41,42]. All these three brain regions belong to the frontoparietal network. The frontoparietal network is an important part of cognitive control network which works in the process of volitional goal-driven behavior in response to changing task demands [43]. The frontoparietal network is prescribed the role of supporting control initiation and providing flexibility by adjusting control in response to feedback. It is hypothesized to be a flexible hub for cognitive control both globally and specifically in terms of distributed connectivity [44-46]. Patients with schizophrenia are characterized by a generalized cognitive deficit with a great deal of evidence that reduced brain activity and connectivity within and between regions of the frontoparietal network was demonstrated across many cognitive tasks [47-49]. After antipsychotic treatment, increased local functional activities in frontal lobe regions of the frontoparietal network and increased functional connectivity with other networks especially the limbic system have been observed [50-52]. As to the right inferior parietal lobule, longitudinal fMRI studies reported that the amount of ALFF in this area and the amount of FC between the bilateral IPLs significantly increased at 1-year follow-up; and increased ALFF in the IPL.R was identified with successful prediction of early treatment in schizophrenia [53,54]. Our study suggested that dynamic brain activities in the regions of frontoparietal network changed in the process of antipsychotic treatment.
The MOG is involved in a visual network (VN) regions focused on visual processing, such as object recognition, perceptual closure and face processing [55]. Dysfunctions in this area may be associated with the neuropsychological mechanism of self-disorder in schizophrenia [56]. Gong et al. [14] conducted a meta-analysis to identify ALFF changes in schizophrenia on the large-scale brain networks. They found that compared to HC, increasing ALFF existed in the VN regions (mainly bilateral occipital gyrus) in first episode schizophrenia and decreasing ALFF was observed in the VN in chronic patients, suggesting an altering activation in the VN regions in patients during different stages of schizophrenia. Hallucination, one of the core symptoms of schizophrenia, associated with the abnormalities in structure and function of visual network which meant a damage of visual information processing [57-60]. Therefore, researchers indicated that the decreased ALFF in the VN in chronic schizophrenia patients linked to the impairment of visual information processing, while increased ALFF in the VN in first-episode patients might be a form of compensation for schizophrenia [14]. Although we did not find abnormal dynamic brain activities in the VN regions at baseline in this study, the dALFF in MOG.R decreased after antipsychotic treatment, which was in line with this assumption to some extent.
The findings of this study provide evidence of abnormal time-varying brain activities in schizophrenia, and support and expand the application of dALFF method in the study of the pathological mechanism in severe mental illness. After 8-week atypical antipsychotics medication, increased dALFF were detected in the frontoparietal network regions and decreased dALFF was found in the VN, which might be related to the imbalance between the advanced and primary functional network. This may reflect the different allocation of brain resources during the process of the disease. The results suggest that atypical antipsychotics may play a therapeutic role by mediating dynamics of intrinsic brain activity in different brain regions and mobilizing the allocation of brain resources from primary brain network to advanced level system. It might be an expression of the strategy of brain resource redistribution in the early stage of schizophrenia. It is notable that in this study, compared to HC, the ITG.R showed increased dALFF in patients at baseline, but after treatment the dALFF value of this area did not change while the dALFF of other areas changed. We infer that perhaps this is not completely intrinsic brain activity normalization, but more of a compensatory effect. The lack of change between pre- and post-treatment in the same brain region may also be affected by a variety of factors such as the small sample size and the uncertainty of the resting state.
There are a few limitations to be noted. First, the sample size of the study was relatively small. Second, the patients were treated with various types of antipsychotics, and we did not perform analyses to separate drug specific effects. Third, the neurocognitive function assessment was not contained in this study, so whether altered brain activity is associated with cognitive dysfunction is unknown. In the future, larger samples and longer-term longitudinal studies are required to investigate the temporal variability of intrinsic brain activities in schizophrenia and the effects of atypical antipsychotics on them.
In conclusion, this study used a sliding-window amplitude of low-frequency fluctuation (dALFF) method to investigate the dynamics of intrinsic brain activity in schizophrenia following antipsychotics. Compared with HC, SZ showed stronger dALFF in ITG.R at baseline. After medication, SZ exhibited reduced dALFF in MOG.R and increased dALFF in SFG.L, MFG.R and IPL.R. In addition, the baseline dALFF in IPL.R was significantly associated with the SANS scores, but the correlation was not observed at following-up. The present study shows that antipsychotics mediate dynamics of intrinsic brain activity in schizophrenia and provides support for the role of dynamic ALFF as a potential biomarker for the pathophysiology of psychosis.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Yingchan Wang. Data curation: Yingchan Wang. Formal analysis: Yuchao Jiang. Funding acquisition: Yingchan Wang, Jijun Wang. Investigation: Jianye Zhang. Methodology: Yingchan Wang, Yuchao Jiang, Dezhong Yao, Cheng Luo. Project administration: Jianye Zhang. Supervision: Jijun Wang. Writing—original draft: Yingchan Wang, Yuchao Jiang. Writing—review & editing: Dezhong Yao, Cheng Luo, Jijun Wang.
Funding Statement
This work was supported by Ministry of Science and Technology of China, National Key R&D Program of China (2016YFC1306800); Shanghai Science and Technology Committee Foundations (16ZR1430500); China Postdoctoral Science Foundation (BX2021078).



