Time to Take Sleeping Pills and Subjective Satisfaction among Cancer Patients
Article information
Abstract
Objective
We investigated the influence of the time to take hypnotics and daytime activity on patient satisfaction with sleeping pills.
Methods
Ninety-six cancer patients who were currently taking benzodiazepine or z-drug as hypnotics were grouped into satisfied and dissatisfied groups. The subjects’ symptoms, time to take sleeping pills, bedtime, sleep onset time, wake up time, and time in bed within 24 hours (TIB/d) were obtained.
Results
The satisfied group had significantly late sleeping pill ingestion time (p=0.04); significantly early wake up time (p=0.01); and significantly shorter sleep latency, TIB/d, duration from the administration of pills to sleep onset, and duration from the administration of pills to wake up time (PTW). Logistic regression analysis revealed that the significant predictors of patient satisfaction to hypnotics were less severity of insomnia [odds ratio (OR)=0.91] and the time variables, including late sleeping pill administration time (OR=1.53) and early wake up time (OR=0.57). Among the duration variables, short PTW (OR=0.30) and short TIB/d (OR=0.64) were significantly related with the satisfaction to hypnotics.
Conclusion
Reducing the duration from the administration of hypnotics to wake up time and TIB/d can influence the satisfaction to sleeping pills.
INTRODUCTION
Insomnia is very common among cancer patients. The traumatic event of being diagnosed with cancer and the cancer treatments, including chemotherapy, surgery, radiation therapy, or hormone therapy, can alter a patient’s sleep-wake cycle. In addition, cancer-related pain, fatigue, vasomotor symptoms, nausea, and vomiting can be causes of sleep disturbance among cancer patients [1-3]. The cognitive-behavior therapy for insomnia (CBT-I) was reported to be helpful in reducing insomnia symptoms, even in cancer patients [4-7]. However, the application of the CBT-I to cancer patients takes time and effort and is not easy for physicians who were not trained.
Physicians can prescribe sleeping pills to patients even when the CBT-I is not available. However, patients sometimes complain that they cannot easily fall asleep despite the intake of sleeping pills. In general, the effect of hypnotics may be influenced by several factors, such as patient’s age, gender, medical or neurologic disease, psychiatric disorders, pain, and fatigue [8]. In addition, we propose that the time of sleeping pill intake should be explored. In our previous two studies [9,10], we observed that the time of sleeping pill administration influenced the patients’ subjective satisfaction to their sleeping pills. Compared with insomnia patients who were not satisfied, those who were satisfied with the hypnotics tended to take it later in the evening, and a 7-hour duration from the administration of pills to wake up time (PTW) was appropriate for their satisfaction [9]. Based on these results, we proposed to instruct the patients to take their sleeping pills seven hours before their wake up time [10]. Although instructing the patients to take their prescribed sleeping pills 30 minutes before sleeping was generally helpful in decreasing their sleep disturbance [11], it may be better for patients to take their sleeping pills at a designated time based on their wake up time.
Cancer-related fatigue, which is one of the most common symptoms of cancer patients, can cause patients to spend a lot of time lying in bed during daytime, may influence the severity of insomnia, and may reduce a patient’s daytime activities [12-14]. Spending a lot of time lying on the bed during the daytime because of cancer-related fatigue may impair the sleep-wake cycle and decrease sleep quality at night [15]. Therefore, we need to assess daytime physical activity of cancer patients when we explore patients’ sleep structure.
The aim of the current study was to explore the association between sleeping pill administration time and the subjective satisfaction to sleeping pills among cancer patients. Furthermore, we explored the influence of TIB/d due to decreased daytime physical activity on their subjective satisfaction to sleeping pills.
METHODS
Subjects and assessments
This study was a retrospective medical records review. The subjects included cancer patients who visited the sleep clinic for cancer patients at the Asan Medical Center, Seoul, South Korea, between January 2017 and June 2018 and who were taking sleeping pills, including benzodiazepine and non-benzodiazepine GABA agonists for insomnia symptoms (ISI>7) [16]. The study protocol was approved by the Institutional Review Board of the Asan Medical Center (2018-0870). All patients were assessed by a psychiatrist and a sleep specialist (S.C.) for their full history and psychiatric examination.
Patients were excluded if they had 1) severe disabilities that can limit the activities of daily living; 2) organic brain disease or cognitive dysfunction; 3) other concurrent sleep disorders, such as restless leg syndrome or periodic limb movements during sleep; 4) snoring or apneic symptoms suggestive of obstructive sleep apnea; 5) experienced cognitive-behavioral therapy for insomnia prior to their visit to our clinic; and 6) intake of other antidepressants or psychotropic medications, such as trazodone or low-dose quetiapine.
The questions that we routinely asked the patients at the first visit [9] included 1) “How many tablets of sleeping pills per day are you taking now?”; 2) “Are you satisfied with your sleeping pills to induce your sleep?”; 3) “What is the usual time to take sleeping pills?”; 4) “What is your usual bedtime?”; 5) “What is your usual time to fall asleep?”; 6) “What is your usual time to finally get out of bed in the morning?”; and 7) “What is the total number of hours you spend laying down within 24 hours, including sleeping time?”. In addition, at the time of clinic visit, the cancer patients were routinely assessed by rating scales, such as the Insomnia Severity Index (ISI) [17,18]; Patient Health Questionnaire-9 (PHQ-9) [19]; Fear of Progression (FoP) [20]; and Cancer-related Dysfunctional Beliefs about Sleep (C-DBS) [21]. We retrospectively reviewed the medical records of all study patients to acquire information that had been routinely obtained at our sleep clinic and their cancer type, stage, and cancer treatment history. We categorized patients into the satisfied and dissatisfied groups, according to their answers regarding the sleeping pills which patients were taking at the first visit.
Calculation of the time and duration variables
We calculated the time and duration variables using patients’ responses to our questions at the first visit (Table 1). The time variables were calculated by averaging the usual times reported in our previous study [9,10]. Using the data, we defined new sleep indices, such as 1) duration from the administration of pills to bedtime (PTB), 2) duration from the administration of pills to sleep onset time (PTS), and 3) PTW. In addition, we gathered information on the total time spent lying down within 24 hours and used it as the TIB/d index.
Calculation of the number of tablets of equivalent hypnotic drugs (TEQ)
In this study, the equivalent dosage of each medication was calculated as alprazolam (0.25 mg), bromazepam (3 mg), clonazepam (0.25 mg), diazepam (5 mg), lorazepam (0.5 mg), triazolam (0.25 mg), and zolpidem (10 mg for the immediaterelease form and 12.5 mg for the extended-release form) [22].
Statistical analysis
Statistical analyses were performed with SPSS ver. 21.0 for Windows (IBM Corp., Armonk, NY, USA). The clinical characteristics were summarized as mean±standard deviation. The level of significance for all analyses was defined as two-tailed p<0.05. A Student’s t-test for continuous variables and chisquare test for categorical variables were done for between group analyses. A Pearson correlation analysis was performed to explore the association among the patients’ clinical characteristics. A logistic regression model was used to explore the variables that may predict the patient subjective satisfaction with sleeping pills.
RESULTS
A total of 96 cancer patients who were taking sleeping pills were included in this study. Among these cases, 50 (52%) subjects reported that they were satisfied with their sleeping pills. No significant differences in gender, age, and psychiatric diagnosis were observed between the groups (Table 1). The sites of cancer were breast (n=32, 33.3%); colorectal (n=12, 12.5%); esophageal and stomach (n=11, 11.5%); lung (n=7, 7.3%); pancreas and biliary tract (n=5, 5.2%); gynecologic (n=5, 5.2%); male urologic (n=4, 4.2%); hematologic (n=4, 4.2%); hepatic (n=3, 3.1%); renal (n=3, 3.1%); thyroid (n=2, 2.1%); and others (n=8, 8.3%). There was no significant difference in the cancer type between the satisfied and dissatisfied groups (p=0.25). Excluding cancers that were not staged using the TNM staging, such as gynecologic cancer (FIGO staging), hematologic cancer, and tumors of the central nervous system, there were no significant differences in the cancer stages between the satisfied and dissatisfied groups (p=0.45). No significant differences between the two groups were observed in the proportions of surgical operation history within the recent three months, chemotherapy, radiation therapy, and antihormonal treatment.
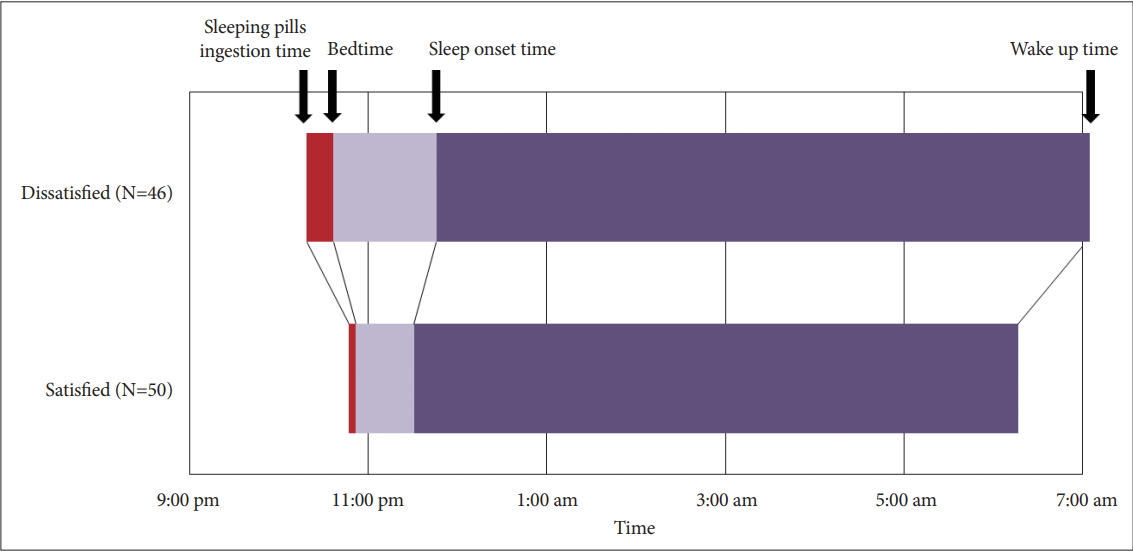
The symptom rating scale scores, including the ISI (p<0.01), PHQ-9 (p<0.01), and FoP (p=0.03) except the C-DBS score, were significantly higher in the dissatisfied group than in the satisfied group (Table 1). Among the time variables, the mean sleeping pill ingestion time was significantly later (10:46±0:59 pm vs. 10:18±1:36 pm, p=0.04) and the mean wake up time in the morning was significantly earlier (6:17±1:18 am vs. 7:02± 1:30 am, p=0.01) in the satisfied group than in the dissatisfied group (Figure 1). All the duration variables, including sleep latency, time in bed, TIB/d, PTB, PTS, and PTW, were significantly shorter in the satisfied group than in the dissatisfied group. No group difference was observed in the number of tablets of equivalent hypnotic drugs.
The severity of insomnia correlated with sleep latency and TIB/d (Table 2). Late sleeping pill ingestion time correlated with a short PTW (p<0.01). A long sleep latency was associated with long PTW and TIB/d (p<0.01 for both), and long time in bed and long PTS was significantly correlated with long sleep latency, PTW, and TIB/d (p<0.01 for all). The number of tablets of equivalent drugs was not significantly associated with sleep latency, PTW, and TIB/d. After adjusting for age, gender, cancer stage, and number of tablets of equivalent drug dosage, logistic regression analysis revealed that among the time variables, less severe insomnia [odds ratio (OR)=0.91, 95% confidence interval (CI) 0.83–0.99]; late ingestion time of sleeping pills (OR=1.53, 95% CI 1.05–2.23), and early wake up time (OR=0.57, 95% CI 0.39–0.83) significantly predicted patient satisfaction with the sleeping pills. Among the duration variables, short PTS (OR=0.30, 95% CI 0.13–0.68) and short TIB/d (OR=0.64, 95% CI 0.51–0.81) were the significant predictors of satisfaction with the sleeping pills (Table 3).

Pearson’s correlation analysis of the age and number of tablets of equivalent hypnotic drugs with the clinical characteristics of insomnia patients
DISCUSSION
In this study on cancer patients, the time of sleeping pill administration was associated with the subjective satisfaction to the sleeping pills. In particular, cancer patients who were satisfied with their sleeping pills tended to take their sleeping pills at a later time and wake up earlier, compared with cancer patients who were not satisfied with their sleeping pills. Moreover, long TIB/d and PTW were associated with patient dissatisfaction with their sleeping pills.
Insomnia is a prominent concern of patients with cancer during cancer trajectories of diagnosis, treatment, and survivorship. When they experience sleep disturbance, they often feel anxious about the negative influence of their sleep disturbance on cancer prognosis [21]. Patients sometimes complain that they cannot easily fall asleep despite the intake of sleeping pills. When hypnotics are not satisfactory, patients often want to increase the dosage to achieve better sleep quality. Physicians also tend to prescribe a higher dosage of hypnotics or switch to other medications when patients report dissatisfaction [9].
Our previous study have shown that earlier intake of sleeping pills from the wake up time in the morning (long PTW) was associated with subjective dissatisfaction with the sleeping pills [9]. In this study, the cancer patients also reported dissatisfaction with their sleeping pills when they took the sleeping pills earlier (10:18 PM vs. 10:46 PM, p=0.04). In our previous study on primary insomnia patients [9], there was no significant difference in the wake up time between the satisfied and dissatisfied groups. However, in this study, the wake up time in the morning was earlier in the satisfied group than in the dissatisfied group (6:17 AM vs. 7:02 AM, p=0.01). Correlation analysis also revealed that short sleep latency was significantly associated with late bedtime, early wake up time, short time in bed, TIB/d, PTS, and PTW. These results implied that a long time spent in bed within the day can be a barrier for satisfaction with sleeping pills among cancer patients.
Insomnia patients usually tend to take sleeping pills and go to bed early in the evening to quickly obtain sleep. Some patients believe that early bedtime can induce early sleep onset. However, on the basis of the two-process model of sleep regulation [23], sleep is regulated by increased sleep debt during wakefulness (homeostatic drive, process S) and sleep-wake cycle related with innate circadian rhythm (circadian timing, process C). Based on our previous study [23], short sleep latency was associated with long duration from wake up time to bedtime (homeostatic drive) and late sleeping pill ingestion (circadian timing). Likewise, cancer patients usually want to take the sleeping pills and go to bed early in the evening in order to have a good rest [12]; however, early ingestion time of sleeping pills cannot guarantee satisfaction to the sleeping pills.
The desire of cancer patients to go to bed early in the evening may be related with their fatigue symptoms, which is common and can impair the circadian rhythm of these patients [24]. We can speculate that the late wake up time in the dissatisfied group may have been from fatigue. We did not measure the fatigue symptom in this study. Rather than, we used the TIB/d, a new index that we used in this study, to assess decreased daytime physical activity of cancer patients. In this study, the TIB/d was significantly shorter in the satisfied group than in the dissatisfied group (9.1 hours vs. 12.4 hours, p<0.001). When we ask patients about their sleep-wake pattern, time in bed during both daytime and nighttime needs to be asked, especially in cancer patients who spend most of their time laying down during daytime due to fatigue.
Depression can also affect fatigue symptoms [25]; in fact, 5.6% to 24.0% of cancer patients suffer from depression [26-28]. Therefore, the concept of primary insomnia may not be fit for cancer patients, and depressive symptoms should be considered as a common comorbidity of insomnia. In this study, 63 (65.6%) patients were diagnosed as having insomnia, and 21 (21.9%) patients had major depressive disorder. In this study, compared with patients with insomnia, those with major depressive disorder had similar in age, gender, cancer stage, time and duration variables, TEQ, and ISI or C-DBS score but had higher PHQ-9 and FoP scores (p<0.001 for both).
This study had several limitations. First, the patients’ subjective satisfaction and their sleep structures were assessed based on their answers to our questions, not on objective assessment tools. Nocturnal polysomnography, which can be useful to rule out occult obstructive sleep apnea or periodic limb movements syndrome, was not performed. Therefore, the other sleep disorders may not have been ruled out. Nevertheless, a psychiatrist and sleep specialist (S.C.) routinely assessed the patients for psychiatric disease and sleep disorders. Second, we could not explore the sleeping pill administration time and satisfaction within a specific cancer type. The prevalence of insomnia and reasons for sleep disturbance vary among the types of cancer. Third, the available information on the type of treatment was based on prior history, not on the ongoing treatment at the time of psychiatric assessment. Because the treatment modalities vary widely among the cancer types, it was not easy to gather this information. In the future, more detailed treatment data in a specified group need to be targeted. Finally, other physical symptoms, such as pain, fatigue, and nausea/vomiting, were not specifically assessed in this study. We tried covering the entire physical activities of the patients using TIB/d, but assessment of the other physical symptoms would have been more helpful.
In conclusion, sleeping pill administration time can affect patient subjective satisfaction with sleeping pills, even among cancer patients. Short PTW and TIB/d were the significant predictors of patient satisfation. Strategies to control and manage cancer patients’ daytime activity and TIB/d need to be developed in order to reduce the use of sleeping pills and improve sleep disturbance.
Acknowledgements
None.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Seockhoon Chung. Data curation: Seockhoon Chung, Soyoung Youn, Byeongil Choi, Suyeon Lee, Changnam Kim. Formal analysis: Seockhoon Chung, Soyoung Youn. Investigation: Seockhoon Chung. Methodology: Seockhoon Chung, Soyoung Youn. Project administration: Seockhoon Chung. Resources: Seockhoon Chung. Software: Seockhoon Chung. Supervision: Seockhoon Chung. Validation: Seockhoon Chung. Visualization: Soyoung Youn. Writing—original draft: Soyoung Youn. Writing—review & editing: Seockhoon Chung, Byeongil Choi, Suyeon Lee, Changnam Kim.