C-Reactive Protein Gene Variants in Depressive Symptoms & Antidepressants Efficacy
Article information
Abstract
Objective
Although the pathogenesis of depression remains unclear, C-reactive protein (CRP) levels are commonly elevated in depressed patients. Thus, CRP single-nucleotide polymorphisms (SNPs) that influence CRP levels may be associated with depression. In the present study, we explored whether CRP SNPs are related to depressive symptoms and antidepressants efficacy in Han Chinese patients.
Methods
We analyzed data from 440 patients with first-episode depression. We obtained genome CRP SNPs, scores of the 17-item Hamilton Rating Scale for Depression 17 (HAMD17) and its four-factor at baseline and after 6 weeks. Quantitative trait analysis was performed using UNPHASED software and curative effects were analyzed using SPSS software.
Results
Male patients with SNP rs1800947G exhibited lower insomnia scores and rs2794521CC exhibited lower scores of anxiety/ physical symptoms, total HAMD17 score. Female patients with rs2794521TT exhibited higher scores of insomnia and lower antidepressants efficacy.
Conclusion
CRP SNPs rs1800947 and rs2794521 may be associated with depressive symptoms in patients with depression in a sex-specific fashion. Furthermore, rs2794521 may be a predictor of the efficacy of antidepressants in female patients.
INTRODUCTION
Depression, the most common mood disorder, is associated with high rates of morbidity, mortality, and disability. Only a small proportion of patients are cured of their depression. Therefore, there is a critical need to develop effective therapeutic strategies and individualized treatment options [1].
Both animal and human studies have revealed that various mood disorders (including depression) induce inflammatory responses, including changes in levels of inflammatory markers and in their responses to psychiatric treatments [2-5]. Such studies have reported that C-reactive protein (CRP) levels in the cerebrospinal fluid (CSF) are elevated in patients with depression [6]. Thus, CRP concentration may be a marker for central and peripheral inflammation, suggesting that CRP plays an important role in the pathogenesis of depression [7-11].
Although accumulating evidence has demonstrated that depressive symptoms and antidepressants efficacy are positively associated with circulating CRP levels, the results of previous studies have been inconsistent [12-17]. Differences in comorbidities, lifestyle factors, and socioeconomic status may explain these discrepancies [18]. Genetic studies may help to clarify these relationships, since the genetic effects are not influenced by such confounding factors. Four CRP single-nucleotide polymorphisms (SNPsrs1130864, rs1205, rs1800947, and rs2794521) have been associated with variations in CRP levels [19,20]. Furthermore, both depression and CRP phenotypes exhibit a genetic tendency of 40% and twin studies have also indicated that depression and inflammation share a common genetic pathway [21].
Despite such evidence, few studies have investigated the relationship between CRP gene polymorphisms and depressive symptoms, with two adult studies reporting no relationship between the CRP gene and depressive or affective symptoms [22,23]. However, another study reported the presence of such a relationship in older adults, but the effects of somatic diseases, i.e., chronic diseases involving cardiovascular abnormalities and/or inflammation, were not considered [24]. Moreover, previous phenotypic studies and etiological studies have suggested that the relationship between the CRP gene and depression may differ between men and women in older age cohorts [19,24]. Despite a dearth of relevant research, some authors have suggested that the CRP gene modifies the relationship between depressive symptoms and CRP levels [23].
Therefore, in the present study, we aimed to more accurately describe the associations between the CRP gene and depressive symptoms/antidepressant efficacy in patients with depression.
METHODS
Participants
We recruited 440 patients (mean age: 34.57±11.97 years; 190 men, 250 women) with first-episode major depressive disorder from the First Hospital of Shanxi Medical University from October 2015 to October 2017. All patients were interviewed by at least two experienced psychiatrists and diagnosed via consensus in accordance with the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [25]. Demographic information, hospitalization history and medication use were carefully documented, and patients with other mental disorders, hereditary diseases, severe somatic diseases, or bipolar disorder were excluded. All patients were of Han Chinese descent and provided written informed consent prior to participation in the study, which was approved by the Institutional Review Board of the First Hospital of Shanxi Medical University (20091217). The 17-item Hamilton Rating Scale for Depression (HAMD17) and its four-factor at baseline were used to determine clinical characteristics. The lowest baseline HAMD17 score among all participants was 17.
Evaluation of antidepressant use and efficacy
A total of 250 patients underwent follow up for 6 weeks. All patients were treated solely with selective serotonin reuptake inhibitors (SSRIs), i.e., escitalopram, paroxetine, sertraline, and fluoxetine at initial doses of 10 mg/d, 20 mg/d, 50 mg/d, and 20 mg/d, respectively. All patients reached the therapeutic dose within 2 weeks of treatment initiation, with a gradual increase of the dose within those 2 weeks. Patients who presented with sleep disorders were prescribed, small doses of benzodiazepines as appropriate (intermittent use, no more than 10 days), and the dosages were adjusted based on clinical symptoms. A total of 223 patients completed 6 weeks of treatment. These patients were divided into a remission group (6-week HAMD17 ≤7) and a non-remission group (6-week HAMD17 >7), into an effective group (reduction rate ≥50%) and an ineffective group (reduction rate <50%).
Genotyping
Haploview software was used to screen genetic loci associated with CRP levels. SNPs rs1130864, rs1205, rs1800947, and rs2794521 were tested for association with variations in CRP concentration. SNP rs2794521 occurred in the promoter region, SNP rs1800947 occurred in the exon 2 region, and SNPs rs1130864 and rs1205 occurred in the 3'untranslated region. DNA was extracted from whole-blood samples obtained from the cubital vein. The genotypes of the four loci were identified via polymerase chain reaction (PCR) and sequencing techniques. The primers were designed using Primer 5.0 software (PREMIER Biosoft Inc., Palo Alto, CA, USA). Primer homology was compared by entering multiple pairs of primers into the BLAST database of the National Library of the United States. The primers with the least homology and suitable Tm values were selected as PCR reaction primers. For genotyping the CRP gene rs1130864, forward primer 5'ACG TTG GAT GAT CTC CAA GAT CTG TCC AAC-3' and reverse primer 5'ACG TTG GAT GTG GGA GCT CGT TAA CTA TGC-3'were used. For rs1205, forward primer 5'ACG TTG GAT GGT TTG TCA ATC CCT TGG CTC-3' and reverse primer 5'ACG TTG GAT GCA GTA GCC ATC TTG TTT GCC-3' were used. For rs1800947, forward primer 5'ACG TTG GAT GGA AAT GTG AAC ATG TGG GAC-3' and reverse primer 5'ACG TTG GAT GCC AGT TCA GGA CAT TAG GAC-3' were used. Finally, for rs2794521, forward primer 5'ACG TTG GAT GCT GAG AAA ATG TGT CCA TGC-3' and reverse primer 5'ACG TTG GAT GCC CTT CCT GTG TCC AAG TAT-3' were used. We utilized a 25-μL PCR reaction system containing the following: 60 ng genomic DNA, 200 μmol dNTPs, 2.5 μL of 10×PCR buffer, 5 pmol of each primer, and 1 unit of TaqDNA polymerase. PCR was performed under the following conditions: pre-denaturation at 95°C for 5 min, followed by 35 cycles at 94°C for 30 s, 63°C annealing for 30 s, 72°C for 30 s, and a final elongation at 72°C for 10 min (supplied by BGI-Huada Genomics Institute, Shenzhen).
Statistical analysis
The goodness-of-fit chi-square test was used to examine Hardy-Weinberg equilibrium for the genotypic distribution of SNPs. UNPHASED software was used to analyze linkage disequilibrium and quantitative traits. Curative effects were analyzed using SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). Genotype frequencies <0.01 were excluded. The level of statistical significance was set at p<0.05 (two-tailed).
RESULTS
General information
Four CRP SNPS exhibited Hardy-Weinberg equilibrium (Table 1). There were no sex-based differences in the distribution of alleles or genotypes at any loci. Linkage disequilibrium (LD) r2 values were as follows: 0.086 (rs1130864-rs1205, D’=-0.822), 0.000 (rs1130864-rs1800947, D’=-0.332), 0.008 (rs113086-rs2794521, D’=0.669), 0.030 (rs1205-rs1800947, D’=-1), 0.212 (rs1205-rs2794521, D’=-0.980), and 0.007 (rs1800947-rs2794521, D’=-1).
CRP gene polymorphisms and depressive symptoms
Quantitative trait analysis revealed no direct association between CRP SNPs and depressive symptoms when all patients were included in the analysis. In this study, association analysis based on gender stratification was carried out. In male patients, we observed significant associations between depressive symptoms and two of the four SNPs (Table 2). Among female patients, only rs2794521 was significantly associated with depressive symptoms (Table 3). Average insomnia symptoms scores were lower in male patients with the rs1800947 G allele than in those with the C allele (Table 4). Less common recessive genotypes were associated with lower scores on items related to anxiety /physical symptoms and total HAMD17 score in male patients with rs2794521 (Table 5). Dominant genotypes were associated with higher insomnia middle scores among female patients in rs2794521 (Table 5). These findings were not observed in the allelic study for rs2794521 when men and women were analyzed separately. The results remained unchanged after 10,000 permutations.
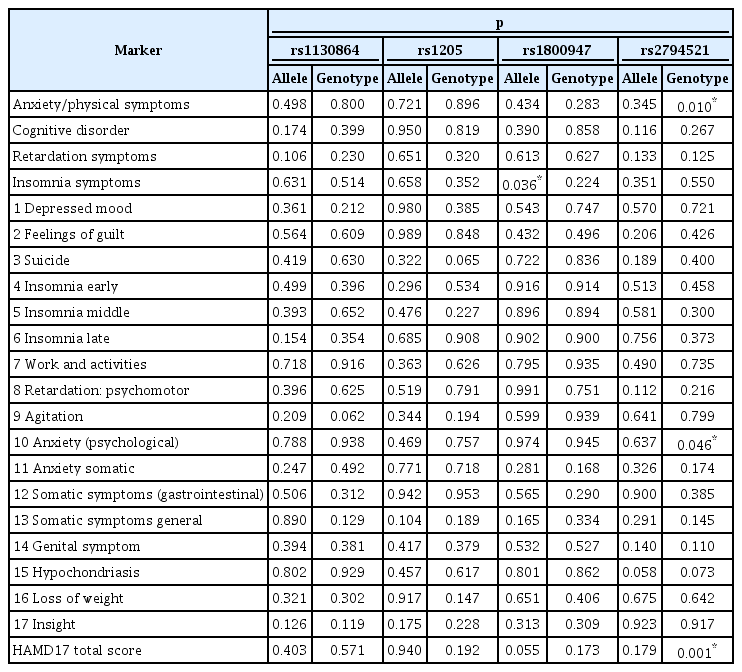
HAMD17 total and clusters scores and the C-reactive protein gene alleles and genotypes of male patients with major depression (N=190)

HAMD17 total and clusters scores and the C-reactive protein gene alleles and genotypes of female patients with major depression (N=250)

Hamilton Rating Scale for Depression 17 cluster scores and the C-reactive protein rs1800947 gene alleles in male patients with major depression
CRP gene polymorphisms and antidepressants efficacy
A rs2794521-sex interaction was found in the effective and ineffective model. In further studies, among female patients, treatment efficacy was lower for patients with the rs2794521TT genotype than for patients with other genotypes (Table 6). Meanwhile, rs1130864-rs1205-rs1800947-rs2794521-sex and rs1130864-rs1205-rs1800947-rs2794521 were not related to antidepressants efficacy (Table 7).

Antidepressants efficacy and the C-reactive protein genotypes/sex of patients with major depression (N=223)
CRP gene polymorphisms, depressive symptoms, and antidepressants efficacy
Among female patients, CRP rs2794521 was associated with both depressive symptoms and antidepressants efficacy. Moreover, in those with the TT genotype, the insomnia middle scores were higher, while the antidepressants efficacy was lower.
DISCUSSION
In the present study, we investigated whether CRP gene polymorphisms were related to depressive symptoms and antidepressants efficacy. However, the relationships between CRP SNPs and depressive symptoms/antidepressants efficacy were not observed uniformly in all patients. Among male patients, rare recessive genotypes or alleles of the CRP gene (rs2794521 CC and rs1800947 G) were associated with significantly lower depressive symptom scores. Among female patients, dominant genotypes were associated with higher mean insomnia scores and lower antidepressants efficacy in those with rs2794521. Since the UNPHASED analysis indicated that rs1205, rs1130864, rs1800947, and rs2794521 were not in the same LD region, haplotype analyses were not performed.
At present, few studies have examined the relationship between CRP SNPs and depressive symptoms, yielding controversial results. In two adult studies, the authors reported no association between the CRP gene and depressive or affective symptoms [22,23]. Luciano et al. [24] adjusted for sex and age in two cohorts of older adults, and reported that rs1130864T and rs1205A were associated with anxiety and neuroticism in female patients. Another [26] group reported an association between rs1130864T and aggressive behavior in women. Kittel-Schneider et al. [27] demonstrated that rs1800947CG and CC were associated with greater depressive symptoms in patients with chronic heart failure. In accordance with these findings, our results suggest that CRP SNPs exhibit sex-specific associations with depressive symptoms. Positive results were observed among male and female patients, and the main loci were rs1800947 and rs2794521. In contrast to previous results, we observed no associations between depressive symptoms and rs1205 or rs1130864 in female patients. Such discrepancies may be explained by the low number of female patients, age differences in subjects among studies, and differences in the research methods utilized. In our study, rare recessive genotypes or alleles of the CRP gene (rs2794521 CC and rs1800947 G) were associated with significantly lower depressive symptom scores in male patients. However, rs2794521 was associated with a similar result in female patients. No previous study has examined associations for rs2794521, which we found to be significantly associated with depressive symptoms even after 10,000 permutations. Indeed, rs2794521 may have functional significance in patients with depression, given that it is located in the 5'promoter region, which is known to regulate gene expression.
In our study, dominant genotypes were associated with lower efficacy of antidepressants in female patients with rs2794521, suggesting that different CRP gene loci are associated with different therapeutic mechanisms. Therefore, our findings have implications for the development of new antidepressants and individualized treatment strategies.
Our study provides new evidence that the CRP gene regulates depressive symptoms and antidepressants efficacy. Indeed, our findings indicated that rs2794521 was associated with depressive symptoms and antidepressants efficacy in female patients. In those with the TT genotype, the insomnia middle scores were higher, while treatment efficacy was lower. We speculate that the TT genotype may regulate antidepressants efficacy via depressive symptoms (Insomnia middle) in female patients with depression. While it must be acknowledged that CRP SNPs may have other functions as well, our results indicate that the high depressive symptom scores may explain the lower reduction rates. In one previous study [22], clinical symptoms were more closely related to metabolic syndrome in patients with the CC genotype of rs1205 than in those with other genotypes. Furthermore, the association between Center for Epidemiological Studies Depression Scale (CESD) scores and CRP levels has been shown to be stronger in individuals with the A-G-T haplotype (rs1417938-rs1800947-rs1205) [23]. However, as we did not measure plasma CRP concentrations, our results cannot be directly compared with these findings.
Numerous studies have suggested a positive correlation between depression and physical disease, indicating that inflammatory factors, such as CRP, may play an important role in the pathogenesis of depression. Increased plasma CRP concentration in patients with depression induces a persistent increase in cortisol concentration [28-30], which further promotes the secretion of inflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) [31], which damages the function of glucocorticoid receptors and causes hyperactivity of the hypothalamic pituitary adrenal (HPA) axis [32]. Further, increased cortisol concentration affects the synthesis of 5 hydroxytryptamine (5-HT) [33]. Together, these responses lead to depression. Therefore, in this study, we examined the efficacy of SSRIs-the first-line treatment recommended for depression-as they act to increase 5-HT levels in the brain. Our findings suggest that efficacy of SSRIs can be predicted based on CRP gene variants.
The gender difference might be attributed not only to CRP, but also to hormones, as CRP fluctuates with sex hormones [34-36]. A previous study found that sex hormones have different effects on HPA axis function [37]. Gender differences also exist in the function of estrogen receptors and glucocorticoid receptors on HPA axis [38,39]. The cortisol/CRP ratio regulates depressive symptoms and may also exhibit gender differences [40]. While we observed sex-specific associations between CRP SNPs and depressive symptoms/ antidepressants efficacy, further studies are required to determine whether these associations are mediated by the HPA axis.
The present study possesses some notable limitations, including the small number of patients with rare genotypes. By further analyzing allelic rather than genotypic effects, we were unable to reach similar conclusions. Therefore, further studies including larger sample sizes are required to verify our hypothesis. In addition, since we utilized a cross-sectional design, we were unable to exclude patients who may have continued to develop bipolar disorder.
In summary, our findings indicate that the CRP SNPs rs1800947 and rs2794521 may exhibit sex-specific associations with depressive symptoms in patients with depression. Furthermore, rs2794521 may be a predictor of antidepressants efficacy in female patients. This is the first study to focus on the relationship between the CRP gene and the efficacy of antidepressant in a population, and the first study on the relationship between the CRP gene and depressive symptoms in a Han Chinese population. Therefore, our results may aid in the development of novel strategies for preventing and treating depression.
Acknowledgements
This study was supported by the National Natural Science Youth Fund Project (81701345, 81601192, 81601193), the National key research and development program of China (2016YFC1307103), National Clinical Research Center on Mental Disorders (2015BAI13B02), National Key Basic Research Program (No.2013CB531305), Application Basic Research Project in Shanxi Province (201801D221418), Shanxi Health and Family Planning Commission Scientific Research Project (201601034), Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi, and Shanxi Province Graduate Student Education Innovation at 2017 (2017BY079). We sincerely thank the patients and their families, as well as the healthy volunteers for their participation, and all the medical staffs involved in the collection of specimens.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Kerang Zhang, Xinxin Li. Data curation: Xinxin Li, Kerang Zhang. Formal analysis: Xinxin Li, Chunxia Yang. Funding acquisition: Ning Sun, Chunxia Yang, Zhifen Liu, Xinrong Li, Kerang Zhang. Investigation: Zhifen Liu, Xinrong Li. Methodology: Ning Sun, Chunxia Yang, Xinxin Li. Supervision: Kerang Zhang. Writing—original draft: Xinxin Li. Writing—review & editing: Ning Sun, Chunxia Yang, Zhifen Liu, Xinrong Li.



