Electroconvulsive Therapy on Severe Obsessive-Compulsive Disorder Comorbid Depressive Symptoms
Article information
Abstract
Electroconvulsive therapy (ECT) is not currently used as a first-line treatment for obsessive-compulsive disorder (OCD). However, several related case reports have demonstrated that ECT seems to be effective for severe OCD, especially when first-line therapies have failed. In this study, we describe the courses, detailed parameters, effects, and follow-up information relating to three patients with severe OCD who were treated by modified bifrontal ECT after their first-line anti-OCD treatments pharmacotherapy, behavioral therapy, and cognitive behavioral therapy failed. The number of ECT procedures administered in each case is as follows: Case 1, eight; Case 2, three; and Case 3, four. In all three cases, the patients' depressive symptoms improved considerably after the ECT procedures. In addition, the condition of all three patients' OCD significantly improved and remained stable at regular follow-ups. ECT may play an effective role in treating severe OCD.
INTRODUCTION
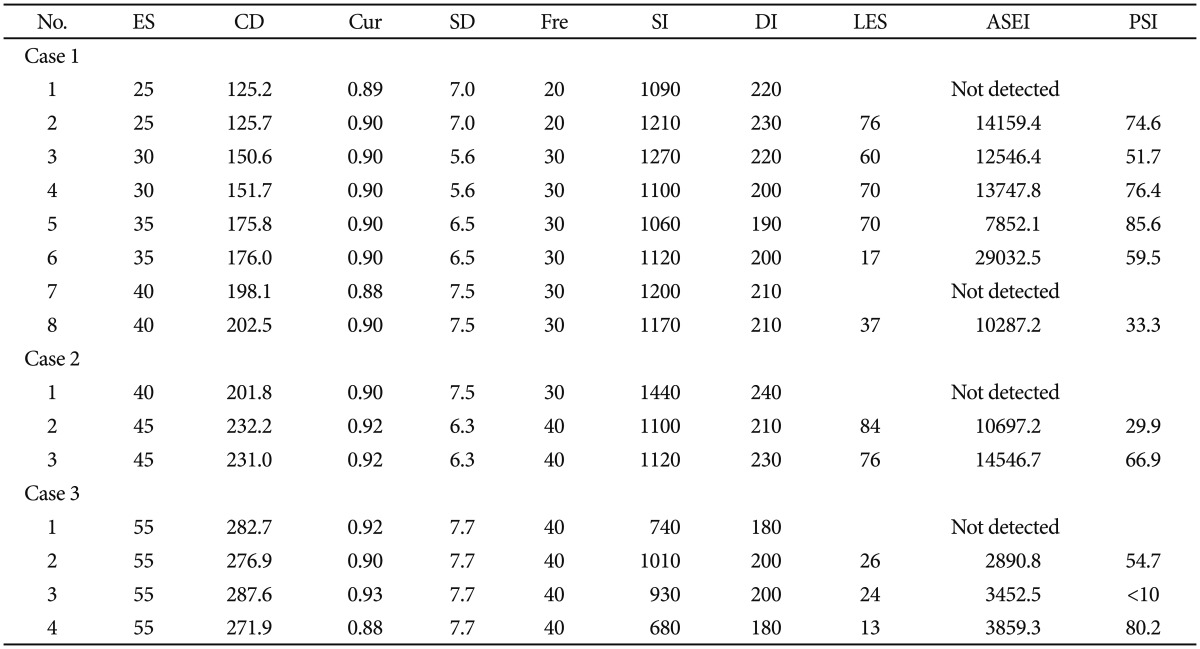
Obsessive-compulsive disorder (OCD) affects numerous people. In fact, approximately 1.9% to 3.3% of the population will experience OCD at some point in their lifetime.1 In the last decade, behavioral therapy (BT), cognitive behavioral therapy (CBT), and medications have been regarded as first-line OCD treatments.2 Yet even after conventional treatment, 25% to 40% of patients have persistent symptoms and lasting functional repercussions2; many patients with severe OCD are still suffering from the condition despite treatment. One nonconventional treatment, called electroconvulsive therapy (ECT), has been found effective for treating severe OCD in a few reports.3,4,5 Yet, evidence describing the relationship between OCD and ECT is still insufficient.6 In this study, we present a case series of three patients with severe OCD who experienced modified ECT (mECT). All patients met the diagnostic criteria for OCD outlined by the World Health Organization in the 10th revision of the International Classification of Diseases (ICD-10). Informed content was obtained from each patient and their legal guardian after the mECT procedures had been fully explained. The modified bifrontal ECT procedure was administered with a Thymatron System IV Integrated ECT Instrument (Somatics, Inc., Lake Bluff, IL, USA), and the parameters of each mECT procedure are presented in Table 1. During each mECT procedure, anesthetic agents varied but mostly involved propofol, succinylcholine, atropine, and 100% oxygen. Results from all three patients' routine examinations at admission were normal and verified the absence of cerebral organic diseases, chromosomal abnormalities, and family histories of mental illness.
CASE
Case 1
Mr. A, at 27 years old, had suffered from OCD for 10 years. He was admitted to the hospital seven previous times because of obsessions, insomnia, depressive feelings, and self-blaming. His previous hospitalizations resulted in pharmacotherapy involving clomipramine, venlafaxine, fluoxetine, and sertraline, but these medications were ineffective. Upon current admission, his scores according to the Hamilton Depression Scale (HAMD) and the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) were 28 and 29, respectively, and he was administered CBT and pharmacotherapy (fluoxetine, 40 mg/d; paroxetine, 20 mg/d; lamotrigine, 50 mg/d; quetiapine, 300 mg/d; and clonazepam, 1 mg/d). Although we changed his medications during the first four months (we prescribed quetiapine, risperidone, clomipramine, olanzapine, etc.), the OCD hardly improved. Therefore, we recommended mECT. Before administering ECT, his HAMD and Y-BOCS scores were 24 and 28, respectively. For the mECT treatment, the patient was administered eight mECT procedures in conjunction with olanzapine (15 mg/d). After the mECT treatments, the patient was treated with Sertraline (150 mg/d) and olanzapine (10 mg/d), and both his OCD symptoms and depressive mood significantly improved. Three days after the mECT procedures, the patient was discharged from the hospital and his HAMD and Y-BOCS scores were 14 and 16, respectively. Mr. A was on a regular follow-up schedule for two years, and his condition remained stable. Follow-up treatments for the patient included seculin at 150 mg/d and olanzapine at 10 mg/d.
Case 2
Mrs. B, a 41-year-old woman, had previously been admitted to the hospital three times and was diagnosed with OCD because of obsessions, compulsions, insomnia, and a depressed mood. Treatments during former hospitalizations involved pharmacotherapies, including clonazepam, clomipramine, sertraline, paroxetine, fluoxetine, etc., which were ineffective. Upon current admission, her Hamilton Anxiety Scale (HAMA), HAMD, and Y-BOCS scores were 16, 29, and 27, respectively. After four days of hospitalization, and given the ineffectiveness of her previously administered medications, the patient and her legal guardians requested mECT. During the three mECT procedures, the patient was given paroxetine (40 mg/d) and zopiclone (500 mg/d) as an adjuvant therapy. The patient experienced one adverse reaction (vomiting) after the third procedure and reported posttreatment agitation three days later that was treated with valproate (250 mg/d). Subsequent ECT procedures were canceled because of the adverse reaction. Pharmacotherapy for the patient included fluvoxamine (100 mg/d) and clonazepam (0.75 mg/d) after the mECT treatments. Her OCD symptoms and depressed mood significantly improved after ECT, and her HAMD, HAMA, and Y-BOCS scores were 11, 10, and 13, respectively. At one years' follow-up, the patient's condition remained stable. The patient was prescribed fluvoxamine at 100 mg/d during the follow-up period.
Case 3
Mr. C, at 53 years old, was admitted to the hospital for obsessions, depressive mood, and insomnia that were caused by business failure. The patient began to wake up early and uncontrollably think about the difficult events he had experienced. Upon admission, his HAMD, HAMA, and Y-BOCS scores were 24, 18, and 21, respectively. He was given maprotiline, which was gradually increased to the maximum therapeutic dose of 225 mg/d after his admission, but his OCD symptoms did not improve. He accepted our suggestion of modified bifrontal ECT. Before ECT, his HAMD, HAMA, and Y-BOCS scores were 19, 20, and 18, respectively. The patient underwent four ECT procedures in conjunction with maprotiline (225 mg/d) and clonazepam (1 mg/d) as an assistant therapy. After four ECT trials, the patient's OCD symptoms and depressive mood improved, and his HAMD, HAMA, and Y-BOCS scores were 11, 9, and 11, respectively, after which the patient canceled further ECT procedures. The patient was given maprotiline (225 mg/d) and clonazepam (0.75 mg/d) after mECT treatment. Nearly four years after he was discharged from the hospital, the patient was in good health and remained symptom free. The patient's drug therapy during the follow-up period was maprotiline at 225 mg/d.
DISCUSSION
The patients' accompanying depressive moods in all three cases improved markedly after the ECT procedures. This result was expected because ECT has already been proven to be an effective treatment for major depressive disorders.7 However, pharmacotherapy and other first-line therapies for OCD rarely improve the symptoms of severe OCD.2 Fortunately, ECT has been shown to be effective for treating severe OCD in several previously published reports.3,4,5 In this article, we presented three patients with severe OCD who were treated with modified bifrontal ECT after first-line OCD treatments failed. Our conservative conclusion is that the three patients' OCD symptoms significantly improved after the mECT procedures. Two patients had no adverse reactions during the mECT treatments, while one patient (Case 2) experienced an adverse reaction and posttreatment agitation.
Many neurological imaging studies on OCD have been presented in the past two decades, showing that some brain regions are closely associated with OCD. These associated brain regions include prefrontal brain regions such as the orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), and the dorsolateral prefrontal cortex (DLPFC). The functional activities in the bilateral, left, or right OFC regions are enhanced in people with OCD,8,9,10 and these changes are often accompanied by increases in bilateral ACC activation.11,12 However, the functional increases in the OFC and ACC are also associated with significant functional decreases in the DLPFC.13 Similar results have also been found in patients with major depressive disorders: studies have shown that, OFC, ACC, and DLPFC dysfunctions and/or structural modifications exist in people with depression.14,15,16 In addition, a recent study found that the average global functional connectivity in and around the left DLPFC is considerably decreased after ECT procedures.17 Consequently, we hypothesized that ECT may play a therapeutic role in treating patients with both OCD and depressive symptoms by influencing the common or related brain regions that are affected by both disorders. Another main hypothesis suggests that ECT partially enhances the transmission of inhibitory neurotransmitters and neuropeptides, such that the active process of inhibiting the seizures caused by ECT is essential to its therapeutic action.18 Therefore, it is possible that an increase in the transmission of inhibitory neurotransmitters and neuropeptides caused by bifrontal ECT acts to "jumpstart" the brain, which is directly related to the observed improvements in OCD symptoms; however, this hypothesis requires further study.
The American Psychological Association (APA) task force on ECT in 1990 stated that when severe depression is prominent, ECT is an effective treatment option for patients suffering from OCD.19 However, until now, this opinion received no support in the form of high-quality evidence from randomized controlled studies. Therefore, our study was designed to add to this body of knowledge and help clarify how ECT affects patients with severe OCD.
Acknowledgments
This research was supported by the National Science & Technology Pillar Program (Grant No. 2013BAI08B03) and the Natural Science Foundation of China (Grant No. 31100812). The funders had no role in data collection, decision to publish, or preparation of manuscript.