Pain in Patients with Dystrophic Epidermolysis Bullosa: Association with Anxiety and Depression
Article information
Abstract
Objective
We investigate the presence and the quality of pain in patients with dystrophic epidermolysis bullosa (DEB), and its correlation with the level of anxiety and depression.
Methods
We collected data from 27 DEB patients and 26 healthy individuals. DEB patients and controls completed 1 scale for the quality of pain, and 1 scale for anxiety and depression. Pain was assessed with the short form of the McGill Pain Questionnaire, whereas anxiety and depression were assessed with the Hamilton rating scale for anxiety and depression.
Results
DEB patients and healthy control individuals were homogeneous for age and gender (p>0.05). A statistically significant difference in the two groups was seen for sensory pain rating scale (p<0.001), affective pain rating scale (p=0.029), total pain rating scale (p<0.001), visual analogue scale (p=0.012) and present pain intensity (p=0.001), but not for anxiety (p=0.169) and depression (p=0.530). The characteristics of pain that showed a significant difference between DEB patients and healthy controls were shooting, splitting, tender and throbbing (p<0.05). In DEB patients pain was not correlated with anxiety or depression (p>0.05), whereas a slight correlation between pain and anxiety was found in healthy controls (p<0.05). No difference was found between quality of pain and anxiety-depression in DEB patients (p>0.05), but was between the DEB dominant and the recessive form of DEB (p=0.025).
Conclusion
The perception of pain in DEB patients appears greater than in healthy individuals, with splitting and tender characteristics being the most significant ones, but was not associated with anxious and/or depressive symptoms.
INTRODUCTION
Epidermolysis bullosa (EB) is a genetic disorder characterized by mucocutaneous fragility with subsequent formation of blisters after friction or mild mechanical trauma.12
EB can be inherited in either autosomal dominant or recessive fashion. There are four major types of EB (and many subtypes) based on the ultrastructural level of separation of the epidermis from the underlying dermis: intraepidermal (Simplex type), intralamina lucida (Junctional type), sublamina densa (Dystrophic type), mixed (kindler syndrome).2
The etiopathogenesis of all forms of EB resides in mutations in more than 10 different genes coding for mutated proteins located at different levels in the epidermis/dermis. Indeed, mutations in Transglutaminase 5, Plakoglobin, Plakiphilin 1 and Demosplakin will be responsible for the suprabasal form of EB Simplex (EBS); mutations in Keratins 5/14, Plectin, BP230, Kindlin-1, Exophilin 5 will be responsible for the basal form of EBS; mutations in Integrins α6β4, Integrin α3, Collagen XVII, and Laminin-322 will be responsible for the Junctional form of EB (JEB), and, lastly, mutations in type VII Collagen will be responsible for both dominant and recessive forms of Dystrophic EB (DEB).2
Due to its multi-systemic involvement and extremely variable clinical phenotype (from very severe and life-threatening to very mild form),1 pain may be a constant and very tough experience for EB patients, mostly for those ones with the more devastating phenotype,3 highly affecting their quality of life.45
Pain is defined by the International Association of the Study of Pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”, 6 and in case of EB it usually derives from cutaneous, or bone/joints, or gastrointestinal involvement as well as surgical procedure.3 Such patients may feel pain due to the constant presence of blisters/erosions on the skin,12 oral cavity,789 ano-genital tract,10 and eyes.11 The feel also pain during their daily activities, like bath, dress changing,12 and urination,3 or during procedures, like hand or esophageal13 as well as cancer resection surgery.3
Therefore, studies on EB patients have become more and more important over time, due to the high impact on their psycho-social life: indeed EB seems to greatly affect not only the quality of life of these patients,45 but also their economic status,14 and their related family members and care-givers' life,15 as well as their psychological asset.16 Nonetheless, different results have been published regarding this last aspect.1718
Because the experience of pain still remains subjective and varies from individual to individual, the management of pain in EB can be complex and challenging12 requiring very often a multidisciplinary approach. It usually includes topical and systemic treatment, and possibly nonpharmacological therapies.3
Over the past few decades, pain has been evaluated via both numerical rating scale19 or visual analogue scale20 or questionnaire21 and the majority of them have mainly addressed the pain management. Therefore, to the best of our knowledge, it seems that no data is currently available in the literature regarding the characteristics of pain in patients with DEB.
As the pain in EB seems to be more representative in patients carrying the dystrophic type,19 we decided to perform a cross-sectional study in order to determine the quality of pain in patients with DEB versus a control group, and correlate it with the level of anxiety and depression.
METHODS
Study design and patients
We collected data from 27 DEB patients and 26 healthy individuals, as a control group, coming from the Epidermolysis bullosa clinic from Dystrophic Epidermolysis Bullosa Research Association (D.eb.R.A.), Mexico. All patients provided their written informed consent. The ethical committee of the Universidad Autonoma de Monterrey (UDEM) approved this cross-sectional study.
We included patients of both gender, aged 16 or older, all race, whose diagnosis of DEB and differentiation between dominant (DDEB) and recessive (RDEB) form were based on clinical criteria and skin biopsy with a routine histology and immunofluorescence antigen mapping, and/or, whereas available, electron microscopy, and/or DNA analysis,2 and were able to give consent if older than 18 years.
Similarly, we included healthy individuals of both genders aged 16 or older, of all races, who worked at the EB clinic as volunteers or were caregivers and who had no other muco-cutaneous diseases, such as lichen planus, eczema, atopic dermatitis, and were able to give consent if older than 18 years. For minor patients, in both groups, consent was provided by their parents or legal guardians.
Exclusion criteria for both groups encompassed patients with psychiatric illness, organic brain syndrome or neurological disease, history of drug abuse, patients regularly treated with psychoneuropharmacological treatment, such as antidepressants, anticonvulsivants, antianxiety or psychotropic drugs or with opioid and non-opioid analgesic medications, because the short form of the McGill Pain Questionnaire (SF-MPQ) is sensitive to clinical changes induced by analgesics.22 Patients were also excluded if they presented with impaired consciousness, any degree of visual impairment, or lack of the necessary communication skills to ensure the reliability of tests.
Two specialists were responsible for every patient who received a medical anamnesis (past, present, family and social), a general medical examination, and an intra and extraoral examination.
Upon admission, specific psychometric instruments (see below) in Spanish versions232425 were employed, which were reviewed for completeness before collection.
Psychometric instruments: evaluation of anxiety-depressive symptoms and self-esteem Short form of the McGill Pain Questionnaire
SF-MPQ22 was used as an index of pain severity and quality. The SF-MPQ is made up of 15 descriptive adjectives for the pain sensation (11 sensory and 4 affective) which were self-rated by the patient according to their intensity level on a point rating scale (0=none, 1=mild, 2=moderate, 3=severe) plus 1 item for present pain intensity (PPI), and 1 item for visual analog scale (VAS).26
In total, we have five scores derived from the SF-MPQ: the Sensory Pain Rating Index (S-PRI), calculated by summing the first 11 pain adjectives (range 0 to 33); the Affective Pain Rating Index (A-PRI), calculated by summing the 4 last pain adjectives (range 0 to 12), and Total Pain Rating Index (T-PRI), calculated by adding together the S-PRI and the A-PRI (range 0 to 45). SF-MPQ also includes the Visual Analogue Scale (VAS), that uses a 10-cm line divided into 1-cm sections, where patients indicate their level of pain by marking the appropriate place on the scale from 0 (no pain) to 10 (the worst possible pain), and the Present Pain Intensity (PPI), where patients indicate their current level of pain ranging from 0=no pain to 5=excruciating. VAS and PPI were both used to provide indices of overall pain intensity.27
SF-MPQ provides a quick assessment when time is limited, taking 2–5 minutes to complete,28 and therefore, this helped in minimizing the respondent burden.
Hamilton rating scale for anxiety
This scale consists of 14 items, each defined by a series of symptoms. It is used to determine the anxiety levels and the distribution of symptoms of patients. Each cluster of symptoms is rated on a five-point scale running from grade 0 (none), 1 (mild), 2 (moderate intensity), 3 (severe), to grade 4 (very severe or grossly disabling), with a total score range of 0–56, with higher scores indicating higher levels of anxiety.29 A score of 7 indicates an absence/minimal anxiety, scores of 8 to 14 represent mild, 15 to 23 moderate, and ≥24 severe anxiety.30
The Hamilton Rating Scale for Depression
The HAM-D contains 21 ratings measured on three (0 to 2) or five (0 to 4) point scales. The first 17 items are used in scoring the instrument. Eight items (4, 5, 6, 12, 13, 14, 16, 17), difficult to quantify, are rated grossly on a 0 to 2 scale: 0=symptom absent, 1=slight or doubtful, and 2=clearly present. Nine items (1, 2, 3, 7, 8, 9, 10, 11, 15) are graded more finely on a 0 to 4 scale in terms of increasing intensity: 0=symptom absent, 1=doubtful or trivial, 2=mild, 3=moderate, and 4=severe. Scores can range from 0 to 52.31 A total score is computed reflecting the degree of symptom severity. Hamilton did not specify cutting-points, but it is generally agreed that scores lower than 7 indicate an absence of depression, scores of 8 to 16 represent mild, 17 to 23 moderate, and ≥24 severe depression.32
Statistic analysis
Descriptive statistics of socio-demographic characteristics of DEB patients and healthy controls were calculated as mean ±standard deviation, frequency, and medians with interquartile range. An exact t test and Pearson's chi-squared test were employed for categorical variables. Difference of medians of all clinical parameters (pain and anxiety) between DEB patients and healthy individuals as well as difference of medians of pain between the types of DEB were calculated by Mann-Whitney U test. The difference in quality of pain was conversely calculated using exact Fisher's test. Linear correlation between quality of pain and anxiety and depression was measured by Spearman's rank correlation coefficient (r). p-values were considered significant if 0.01<p≤0.05 and highly significant if ≤0.01.
RESULTS
Socio-demographic and clinical characteristics
We enrolled 27 patients (9 males and 18 females) with DEB in the study with mean age of 31.5±11.4 and 26 healthy individuals (9 males and 17 females) with mean age of 30.4±9.6. No statistical difference was noted between DEB patients versus control group in terms of age (p=0.697), gender (p=0.922), anxiety (p=0.169) and depression (p=0.530). In the group of DEB patients, the majority of patients were affected by the severe generalized recessive form (51.9%) (Table 1).
Pain perception and quality of pain
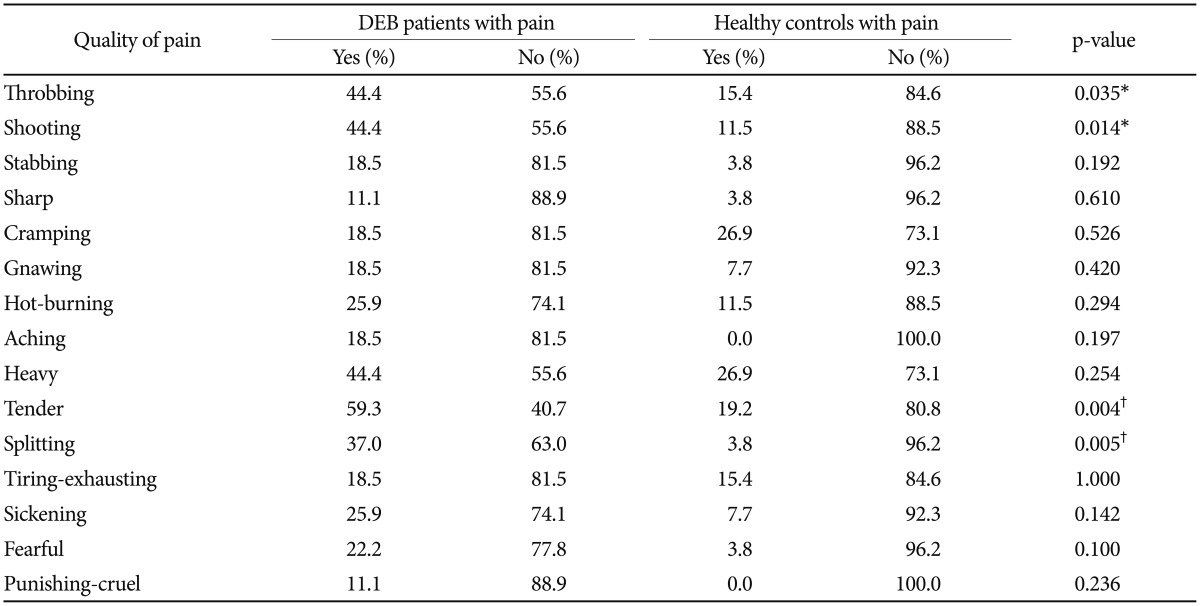
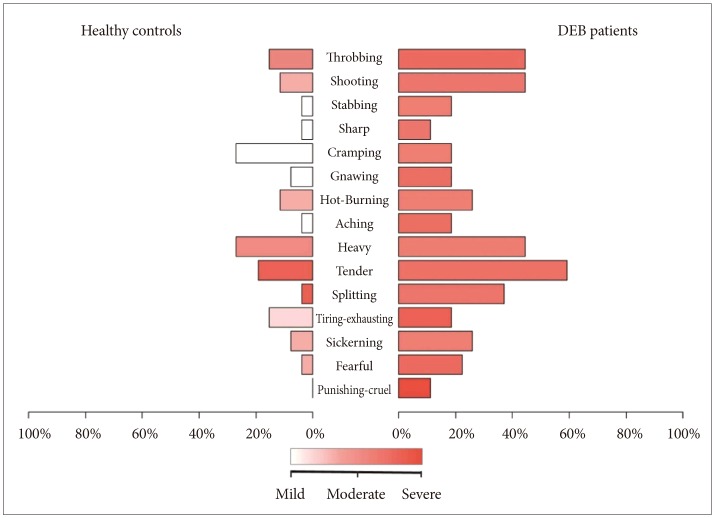
The analysis of pain measured with SF-MPQ revealed that DEB patients perceive greater pain than healthy individuals in terms of intensity (VAS, p=0.012 and PPI, p=0.001) (Table 1, Figure 1) and quality of pain sensory scale (S-PRI) (p<0.001) and affective subscale (A-PRI) (p=0.029) (Table 1, Figure 2). Among all different qualities of pain, a difference between the two group was only seen in throbbing (p=0.035), shooting (p=0.014), tender (p=0.004), and splitting (p=0.005) (Table 2, Figure 3).

Comparison of intensity of pain via present pain intensity and visual analogue scale between dystrophic epidermolysis bullosa patients and healthy controls.
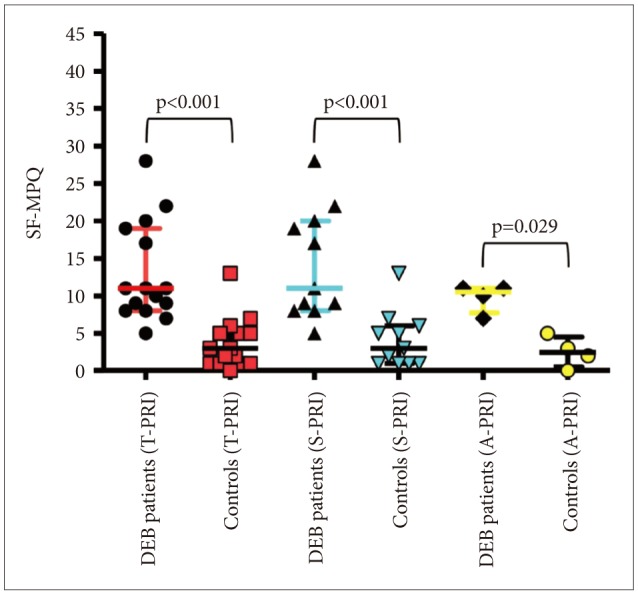
Comparison of quality of pain via sensory, affective, and total pain rating index between DEB patients and healthy controls (p<0.05, Mann-Whitney U test). SF-MPQ: short form of the McGill Pain Questionnaire, DEB: dystrophic epidermolysis bullosa.
Correlation of pain with and anxiety/depression
The analysis of correlation of VAS and PPI with HAM-A and HAM-D showed that in the DEB patients VAS and PPI correlate with each other (p<0.001), but they do not correlate with anxiety and depression (p>0.05), whereas anxiety does correlate with depression (p<0.001). On the other hand, in the control group, VAS and PPI seems to slightly correlate with anxiety (p=0.020 and 0.032, respectively) (Table 3). Additionally, the analysis of correlation between each single quality of pain and anxiety and depression failed to demonstrate any statistical significant result (p>0.05) (Table 4).
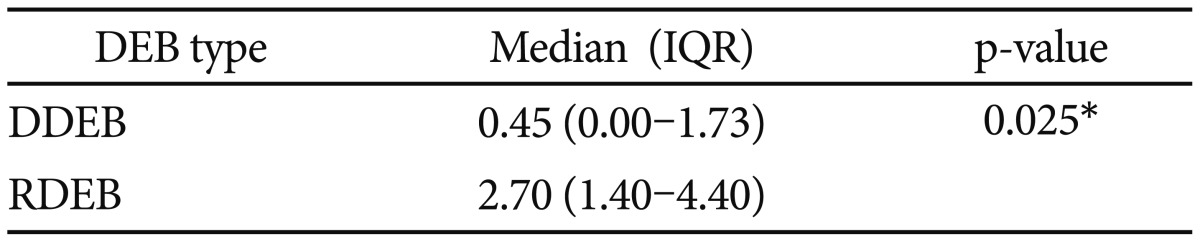
Lastly, level of pain measured by VAS showed a difference between the dominant and recessive form of DEB (p=0.025) (Table 5).
DISCUSSION
Pain plays a major role in EB patients' life, representing a tough challenge, as it may be constant even during routine daily activities.
Several papers have been published over the past 20 years regarding the pharmacological and non-pharmacological management of pain in EB,31213 but no data about the characteristics of pain in patients with EB is currently present in the literature. Therefore, this seems to be the first cross-sectional study reporting on the quality of pain in a cohort of patients with one of the three major types of EB, the dystrophic one, in an attempt to provide additional information on how to better manage pain in DEB patients.
We analyzed a sample of 27 patients with DEB and 26 healthy individuals, that turned out to be homogenous in terms of age, gender and anxiety and depression status (p>0.05) (Table 1).
The quality of pain in patients with DEB was measured using a well-known and validated tool, SF-MPQ.
In patients with DEB, SF-MPQ showed that both the sensory and affective components were more impaired than in the healthy controls (p<0.001 and p=0.029, respectively). Similarly, even the intensity of pain measured with VAS and PPI showed a statistical significant difference between DEB and healthy individuals (p=0.012 and p=0.001, respectively) (Table 1). However, interestingly, the level of pain in DEB was not so high as expected with a median of 1.80 (range, 0.10–4.00) for VAS and a median of 1.00 (range, 0.00–2.00) for PPI (Table 1). The stratification by DEB form demonstrated that the level of pain measured with VAS was much higher in patients with the recessive form than in those with the dominant one (median 2.70 vs. 0.45, respectively; p=0.025) (Table 4), although the sample size between the 2 forms was not homogenous (Table 1).
We also calculated the presence/absence of a specific type of pain showing difference in intensity between the 2 groups (Figure 1). Our analysis demonstrated that only 4 different quality of pain (tender, splitting, throbbing, and shooting) were significant between DEB patients and healthy individuals (Table 2). No statistical analysis for intensity of pain was performed because the sample size was too small. These results are probably not surprising given the nature of this disease. Indeed, the highly significant difference into the sensory category (tender and splitting) might be explained by the fact that the skin in DEB patients is too thin or even absent, making this kind of pain predominant. Interestingly, DEB patients report a perception of pain also into the temporal category (throbbing) and into the spatial category (shooting), meaning that their pain can be waxing and waning and so severe to start in one place then quickly move to another.
As chronic pain condition can influence mood states, leading to the development of depressive symptoms,33 we decided to measure the level of anxiety/depressive symptoms in DEB patients, trying to correlate them with the intensity of pain. As different scales for anxiety and depression have demonstrated to provide the same information,18 we decided to choose just one, that was, Hamilton for anxiety and depression administered by a physician with a background in psychotherapy.
The analysis of intensity of pain and psychometric battery scales failed to demonstrate any correlation between pain and anxiety/depression in DEB patients (p>0.05), unlike healthy individuals, where a weak-but statistically significant-correlation was found between pain and anxiety (Table 3). We do not know why we obtained this paradoxical finding; we may hypothesized that 2 factors were implicated: 1) the cross-sectional nature of the study and 2) the presence of pain since birth for the majority of DEB patients, making them used to it. Therefore, they might have a less likelihood of developing anxiety because of pain.
Therefore, we may conclude that DEB patients experience more pain than healthy individuals with splitting and tender characteristics being the most significant ones and that pain in DEB patients does not correlate with anxious/depressive symptoms.
It is also important to highlight that our results showed some limitations including the small sample size, the restriction to just one EB type, and the analysis of the psychological (anxiety and depression), the sensory and affective aspect of the pain (SF-MPQ) only. In fact, we now know that pain is widely conceived as a biopsychosocial phenomenon that is influenced not only by biological (e.g., injury, infection), psy-chological (e.g., negative mood), but also by cognitive, behavioral and socio-ethnocultural factors, that can dynamically interact to influence a person's overall experience of pain.34
Additionally, we need to underline that all DEB patients were coming from Northern Mexico and were young adults over the age of 16, whose level pain was unexpectedly quite low and easily manageable with acetaminophen even for those activities considered to be the most painful ones, like bath and changing bandages. However, we do not know whether we could get the same results from an EB pediatric population. This aspect should be further investigated by using appropriate psychometric scales designed for pediatric patients to test both anxiety/depression, like the Revised Child Anxiety and Depression Scales,35 and intensity/quality of pain, like the faces pain rating scale, the colored analogue scale,36 or the cloudy ranking scale.37
Due to the small sample size, the relatively low level of anxiety and depression with unexpectedly quite low level of pain, our results should be interpreted with caution. Therefore, it would be interesting to perform multicenter studies in different countries of the world on a larger sample of patients with different types of EB, different ethnic and genetic background, in order to confirm our results.
We believe that the evaluation of the quality of pain was the most important and unique aspect of our study, as this might have some implications in driving our therapeutic choice when managing pain in DEB patients. The main goal would be to try to understand whether or not we might have a more targeted pain management based on a specific type of pain. Indeed, pain management includes different therapeutic options, not just opioid analgesics that are widely used in EB patients.38 Whether these other options, such as anticonvulsants and antidepressant, may be employed to obtain a better analgesia in patients with EB patients, mostly RDEB, still remains an open question and, therefore, clinical trials, possibly randomized controlled ones, should be performed in the near future.
Acknowledgments
We are very grateful to all DEB patients for their courage, strength, and love. We would like to thank all DebRA Mexico, Monterrey, particularly Lic. Erika Salas for her valuable and priceless assistance.