Effects of Antidepressant Treatment on Sexual Arousal in Depressed Women: A Preliminary fMRI Study
Article information
Abstract
Objective
There was a recent study to explore the cerebral regions associated with sexual arousal in depressed women using functional magnetic resonance imaging (fMRI). The purpose of this neuroimaging study was to investigate the effects of antidepressant treatment on sexual arousal in depressed women.
Methods
Seven depressed women with sexual arousal dysfunction (mean age: 41.7±13.8, mean scores of the Beck Depression Inventory (BDI) and the 17-item Hamilton Rating Scale for Depression (HAMD-17): 35.6±7.1 and 34.9±3.1, respectively) and nine healthy women (mean age: 40.3±11.6) underwent fMRI before and after antidepressant treatment. The fMRI paradigm contrasted a 1 minute rest period viewing non-erotic film with 4 minutes of sexual stimulation viewing an erotic video film. Data were analyzed by SPM 2. The relative number of pixels activated in each period was used as an index of activation. All depressed women were treated with mirtazapine (mean dosage: 37.5 mg/day) for 8 to 10 weeks.
Results
Levels of brain activity during sexual arousal in depressed women significantly increased with antidepressant treatment (p<0.05) in the regions of the hypothalamus (3.0% to 11.2%), septal area (8.6% to 27.8%) and parahippocampal gyrus (5.8% to 14.6%). Self-reported sexual arousal during visual sexual stimulation also significantly increased post-treatment, and severity of depressive symptoms improved, as measured by the BDI and HAMD-17 (p<0.05).
Conclusion
These results show that sexual arousal dysfunction of depressed women may improve after treatment of depression, and that this improvement is associated with increased activation of the hypothalamus, septal area, and parahippocampal gyrus during sexual arousal.
INTRODUCTION
Depressed patients often have difficulties in sexual function including loss of sexual interest, and disturbance of sexual arousal or orgasm.1-8 There have been very few studies, however, of neural mechanisms of sexual dysfunction in depressed patients.
We previously demonstrated the functional neuroanatomy associated with sexual dysfunction in depressed men by using functional magnetic resonance imaging (fMRI) with a visual sexual stimulation paradigm.9 For depressed men, regional brain activation during sexual arousal was found to be significantly decreased, in comparison to healthy men, in the regions of hypothalamus, thalamus, caudate nucleus, and inferior & superior temporal gyri.9
Recently we also demonstrated the functional neuroanatomy associated with sexual dysfunction in depressed women.10 Regional brain activity in depressed women during visual sexual stimulation was significantly less than that of healthy women in the regions of the hypothalamus, septal area, middle occipital gyrus, middle temporal gyrus, anterior cingulate gyrus, inferior frontal gyrus, parahippocampal gyrus, and thalamus. These decreases in brain activation were more than 50% lower in the brain regions of the hypothalamus, septal area, anterior cingulate gyrus, and parahippocampal gyrus.
This study represents the first clinical report of the effects of antidepressant treatment on brain activation response to sexual stimuli in depressed women using a blood oxygenation level dependent (BOLD) fMRI technique. Because many antidepressants such as selective serotonin reuptake inhibitors (SSRIs) may induce sexual dysfunction,11 for this study we treated depressed women with mirtazapine which has been reported to be less associated with sexual dysfunction than SSRIs.12
METHODS
Subjects
Seven right-handed female depressed women with major depressive disorder (MDD) and sexual arousal dysfunction participated in this study. They ranged in age from range 21 to 59 (mean 41.7 years) and had a mean Beck Depression Inventory (BDI)13 score of 35.6±7.1 and a mean 17-item Hamilton Rating Scale for Depression (HAMD-17)14 score of 34.9±3.1. And nine right-handed healthy women (age range 23-58: mean 40.3 years), who were gender- and age-matched, participated. All participants gave written informed consent and were asked to avoid sexual contact leading to orgasm for at least 24 hours before fMRI. They did not take any psychotropic medications before treatment. Diagnostic evaluations for the MDD subjects used the Structured Clinical Interview for the DSM-IV.15 Depression severity was assessed with the BDI and HAMD-17. Sexual arousal dysfunction was diagnosed using the DSM-IV criteria A and B for female sexual arousal disorder.15 The study protocol was approved by the Ethics Committee of Chonnam National University Hospital.
Treatment
All depressed patients were treated with mirtazapine (mean dosage, 37.5 mg/day) and as-needed benzodiazepines for 8 to 10 weeks.
Sexual stimulation paradigm
To identify the brain region activations associated with sexual response, visual stimuli in the form of erotic and non-erotic films were presented in real time. Stimuli were silent video films that were presented to the subjects through a mirror located at the top of the head coil that received video-images from outside of MR scanner room. The visual sexual stimulation paradigm consisted of two cycles with single periods of rest and activation: Each began with a 1 minute rest period presentation of a non-erotic documentary film, followed by a 4 minute activation period with an erotic video film presentation.
Immediately after each fMRI acquisition, the participants were asked to report "To what degree were you aroused?" on a scale of 1 (no sexual arousal) to 10 (maximal sexual arousal).
fMRI acquisition and data analysis
T1-weighted images (TR/TE/flip angle=500 ms/9 ms/90°) were obtained to depict the anatomy using a 1.5T MRI scanner (GE Medical System, Milwaukee, WI, USA). The BOLD-based functional images were acquired from 10 slices parallel to the anterior commissure and posterior commissure lines using a gradient-echo EPI pulse sequence (TR/TE/flip angle=3,000 ms/50 ms/90°) with 6 mm slice thickness and a 1 mm gap. Image reconstruction was performed on "MRIcrow 1.36" (Univ. Nottingham) and SPM 2 (Welcome Department of Cognitive Neurology, London, UK) programs to realign and spatially normalize the images to a template brain that approximates Talairach atlas spaces. Images were then smoothed with a Gaussian spatial filter at a full-width-at-half-maximum of 8 mm to increase signal to noise ratio.
Statistical activation maps were reconstructed from the contrast of erotic video film vs. non-erotic documentary film using two-sample t-test. The mean-images of both the healthy and depressed women were obtained using one sample t-test, and were identified by whole-brain analysis with clustering threshold of 27 minimal size and a statistical threshold of p<0.05. We quantified the regional brain activity (%) using the program FALBA (functional and anatomical labeling of brain activation).16 Brain activity (%) was defined by the percentage of the activated voxels out of a total number of voxels consisting of a given anatomical area. Paired t-tests were used to compare the differential brain activities (%) between before and after treatments in depressed women (p<0.05).
RESULTS
Depressive symptoms
Depressed symptoms of all participant patients were improved: the scores on the BDI (11.9±8.2) and HAMD-17 (9.7±6.3) were significantly decreased with antidepressant treatment (p<0.05).
Self-reported sexual arousal
With an antidepressant treatment, the 10-point scale for self-reported sexual arousal during visual sexual stimulation in depressed women was significantly enhanced to 5.7±0.7 from 3.9±0.8 (p<0.05).
Brain activation
Figure 1 shows the fMRI images obtained from one sample t-test for healthy women and depressed women before and after treatments. The cerebrocortical regions, which were significantly activated by visual sexual stimulation, were the hypothalamus, septal area, middle occipital gyrus, middle temporal gyrus, anterior cingulate gyrus, inferior frontal gyrus, parahippocampal gyrus, thalamus, amygdala, and insula in healthy women. Brain activities (%) associated with visual sexual stimulation in depressed women were significantly enhanced with antidepressant treatment in the regions of the hypothalamus (3.0% to 11.2%), septal area (8.6% to 27.8%) and parahippocampal gyrus (5.8% to 14.6%) (p<0.05) (Figure 2).
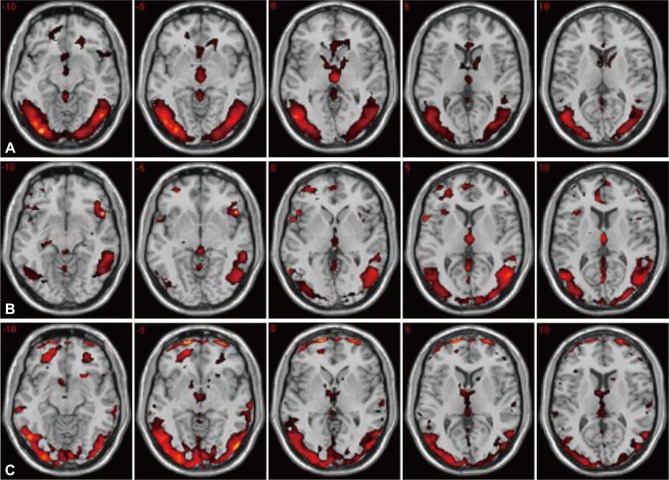
A series of the axial fMR images activated by visual sexual stimulation in healthy (A) and depressed women before (B) and after (C) antidepressant treatment, resulting from the one sample t-test (p<0.05).

Comparison of the brain activities during visual sexual stimulation in healthy and depressed women before and after antidepressant treatments. Paired t-test was used to compare the differential brain activities (%) between before and after treatments in depressed women. The brain areas marked with asterisks (*) indicate the significant change of brain activity after treatment (p<0.05). HTHL: hypothalamus, SEP: septal area, MOG: middle occipital gyrus, MTG: middle temporal gyrus, aCIN: anterior cingulate gyrus, IFG: inferior frontal gyrus, PHIP: parahippocampal gyrus, THL: thalamus, AMY: amygdala, INS: insula.
DISCUSSION
These findings demonstrate significant changes in sexual arousal and brain activation to sexual stimulation after antidepressant treatment in depressed women. After antidepressant treatment, subjective levels of sexual arousal were significantly improved and the brain activation of hypothalamus, septal area and parahippocampal gyrus regions were significantly increased during visual sexual stimulation.
In this study, mirtazapine was used to treat depression. Mirtazapine is a noradrenergic and serotonergic antidepressant. The enhancement of serotonergic neurotransmission is specifically mediated via 5HT1 receptors because mirtazapine is a postsynaptic serotonergic 5HT2 and 5HT3 antagonist.17 Clinical researches have shown that mirtazapine has an overall efficacy similar to that of tricyclic antidepressants, but with relative absence of cholinergic, adrenergic, and serotonergic side effects.18 Some studies have reported that for patients who have a satisfactory antidepressant response to SSRIs but experience sexual side effects, discontinuing the SSRIs and initiating medication of mirtazapine often can provide continuing remission of depression and returning of satisfactory sexual functioning.12,19 Recently, Kim and their colleagues suggested that the different rates of sexual side effects between the patients in the SSRI-treated group and the mirtazapine-treated group may be due to different effects on brain activation.20 Although 5HT2 and 5HT3 antagonism of mirtazapine is thought to contribute to less sexual dysfunction, the precise mechanisms are not yet clear understood and need to be further studied in the future.
In order to explore the central nervous system mechanism of sexual arousal, functional imaging techniques have been utilized.21-26 Stoleru21 and Redoute26 used positron emission tomography (PET) to investigate changes in regional cerebral blood flow corresponding to visual sexual stimuli in healthy subjects. They reported that activation of the right orbitofrontal cortex was correlated with both the cognitive and motivational components of the proposed model. They also suggested that activation of the rostral portion of the anterior cingulate cortex and the posterior portion of the hypothalamus was correlated with the autonomic component of sexual arousal whereas activation in superior and middle frontal gyri and anterior cingulate gyrus was related to the level of perceived emotion. We previously used BOLD fMRI technique to evaluate the cerebral centers associated with sexual arousal response in healthy subjects during viewing of erotic films.22,24 Functional MRI is a non-invasive technique that is not limited in the number of scans with no exposure to radioactive pharmaceuticals.27 The brain activation patterns in healthy male and female volunteers were similar to those of previous PET studies.21,25
In addition to studies with healthy subjects, our two recent studies also reported a significant difference of brain activations between healthy and depressed patients during sexual stimulation using fMRI.9,10 For male depressive patients, the level of activation was significantly less than that of healthy men in the hypothalamus, thalamus, caudate nucleus, and inferior & superior temporal gyri.9 For female depressed patients, the following cortical areas showed decreased brain activity on sexual arousal: hypothalamus, septal area, anterior cingulate gyrus, and parahippocampal gyrus.10
This study further supports the association of hypothalamus, septal area, and parahippocampal gyrus with sexual arousal of depressed women by showing that the partial recovery of brain activation to sexual stimulation after antidepressant treatment. This is consistent with other evidence for these regions as the core centers for sexual arousal and the regulation of sexual behaviors in humans.
The hypothalamus has been well-known to play a pivotal role in the regulation of sexual behavior and physiological arousal.26,28-31 The greater hypothalamic activation we previously reported in healthy women relative to untreated depressed women could be viewed as suggesting that healthy women were physiologically more aroused than depressed women in response to the visual sexual stimulation. And this result suggests that low activation of the hypothalamus may be a mechanism by which depression results in sexual dysfunction.
The septal area is known for regulating active sexual behavior.32 Rats with septal lesion suppressed the performance of mounts, intromissions, and ejaculations of sexual behaviors.32 Less activation of septal area in depressed women is related to suppression of active sexual urge in depressed state.
The parahippocampal gyrus was also significantly less activated region in depressed women than in healthy women. Previous research has shown that this area has an important role for expression of individual recognition in the context of the Coolidge effect.33 The Coolidge effect is a phenomenon in which males show renewed sexual interest in a novel female following copulation to satiety with another female.34 A recent human fMRI study suggested that this region might be related with emotional memory of sexual behavior.35
In this study, we carried out, for the first time, correlative studies on after-treatment response about the functional neuroanatomy of the brain associated with female sexual arousal in depressed women by using BOLD-based fMRI. Limitations in this preliminary study were the small sample size and the lack of objective physiological measures of sexual arousal. In the absence of a placebo group, it cannot be ruled out that changes in regional activations were due to time alone.
In the future, more systemized studies with a larger sample of subjects and a combined objective physiological measure of sexual arousal would be necessary to gain more informative results. Furthermore, research on other psychiatric illnesses with sexual dysfunction and exploration of gender difference will be needed to clarify the specificity of these findings to depression and to women, respectively.
In conclusion, this study showed that increased activation of hypothalamus, septal area, and parahippocampal gyrus are associated with treatment-related improvement of sexual arousal in depressed women with sexual arousal dysfunction. The importance of these regions is supported by prior findings implicating the same regions in sexual arousal dysfunction among untreated women with depression. The findings in this study may help advance understanding of neural mechanisms of human sexual response and pathophysiology of sexual dysfunction in depression.
Acknowledgments
This paper was supported by research funds of Chonbuk National University in 2009. We also wish to thank Franklin Schneier, professor of Columbia University, who provided helpful comments on the draft.