Gamma Oscillation in Schizophrenia
Article information
Abstract
Dysfunctional neural circuitry has been found to be involved in abnormalities of perception and cognition in patients with schizophrenia. Gamma oscillations are essential for integrating information within neural circuits and have therefore been associated with many perceptual and cognitive processes in healthy human subjects and animals. This review presents an overview of the neural basis of gamma oscillations and the abnormalities in the GABAergic interneuronal system thought to be responsible for gamma-range deficits in schizophrenia. We also review studies of gamma activity in sensory and cognitive processes, including auditory steady state response, attention, object representation, and working memory, in animals, healthy humans and patients with schizophrenia.
INTRODUCTION
Individuals with schizophrenia show diverse symptoms and deficits in multiple domains of perception and cognition.1-3 These include cognitive disturbances, such as attention deficits and delusional ideation; disturbances of self-awareness and agency; alterations in emotional expression; disturbed motor behavior; and sensations in the absence of external stimulation, or hallucinations. These varied abnormalities in schizophrenia may be due to a dysfunction in neural circuitry, which affects many brain systems, rather than to a lesion affecting a localized site. One model that has garnered much attention in the past decade is the "disconnection hypothesis," which proposes that schizophrenia disrupts signaling among brain regions, systems or cellular circuits.4,5 As early as a century ago, Wernicke hypothesized that psychosis was caused by a pathology of association fibers.6 Recently, diffusion tensor im-aging has suggested that changes occur in the integrity of the white matter tract in schizophrenia,7 findings are supported by post-mortem analysis of white matter pathology.8,9 Deficits in functional connectivity have been consistently observed in schizophrenia, as shown by reduced interregional correlations of positron emission tomography and functional magnetic resonance imaging signals. Functional connectivity has been reported to affect the prefrontal, temporal, cingulate and parietal cortices in individuals with schizophrenia.10-12
Functional dysconnectivity may result from structural alterations in axonal integrity, differences in mapping of projections among brain regions or physiological disturbances of neurotransmission. Disruption of neural synchrony and oscillations, for example, could have a marked impact on functional connectivity within and across brain regions. Findings from cellular, local field potential, and electroencephalographic recordings suggest that gamma oscillations (>30 Hz) are important for integration of information within neural circuits. Gamma oscillations in the neural system were first reported in the olfactory nerves of hedgehogs, which in response to olfactory stimulation, produced trains of sinusoidal oscillations in the gamma frequency band.13 More recently, gamma oscillations have been associated with numerous perceptual and cognitive processes, including attention,14-17 memory,18,19 object recognition,20 word processing,21,22 and consciousness.23
Electroencephalography (EEG) and magnetoencephalography (MEG) have shown that schizophrenia is characterized by alterations in synchrony and oscillatory activity in a variety of paradigms, particularly in the gamma range. These disturbances could have pervasive effects on cognitive function. This review will provide an overview of the neural basis of gamma range oscillations, methods of eliciting and measuring gamma activity in humans and animals, evidence for the roles of gamma activity in sensory and cognitive processes, and findings in schizophrenia.
CELLULAR MECHANISMS SUPPORTING GAMMA OSCILLATIONS
Gamma oscillations have been observed in many cortical regions and networks, including visual,24,25 auditory,26 motor,27 parietal,19 and hippocampus.28,29 Due to its simple laminar structure, which generates higher gamma power than other cortical areas, the hippocampus has been most frequently used in investigations of the cellular mechanisms underlying the generation of gamma oscillation.30 The first in vitro demonstration that networks of inhibitory gamma-aminobutyric acid (GABA) interneurons generated gamma oscillations were in rat hippocampal specimens.31 Tetanic stimulation evoked gamma oscillation in the CA1 region, even in the presence of glutamate receptor inhibitors, suggesting that excitatory neuronal function might not be necessary for gamma oscillations.31 In contrast, gamma oscillations were totally blocked by the GABA type A receptor antagonist bicuculline, suggesting that GABAergic interneurons are essential in generating gamma oscillations.31 Moreover, single GABAergic interneurons were observed to synchronize the firing of a large number of pyramidal cells, due to the divergence of outputs from these GABAergic interneurons.32
GABAergic interneurons are present throughout the cerebral cortex, extending to all layers and constituting approximately about 25-30% of the neuronal population in primate neocortices.33,34 These interneurons can be categorized according to their electrophysiological characteristics, as fast-and non-fast-spiking interneurons; according to their formation of synapses with other neurons, as soma-inhibiting and dendrite-inhibiting; or immunocytochemically, according to their localized expression of calcium-binding proteins, such as parvalbumin, calretinin, and calbindin.35 Among those subpopul-ations, GABAergic neurons that express parvalbumin were found to generate gamma oscillations in vivo and in vitro.36,37 Stimulation of parvalbumin GABAergic interneurons was found to increase gamma oscillations, whereas inhibition of these interneurons suppressed gamma oscillations.37 However, even if GABAergic interneurons are primarily responsible for gamma oscillations, pyramidal neurons, the principal class of excitatory neurons, are required to induce long-range gamma synchronization in networks that exceed the limited spatial projections of GABA neurons. Pyramidal neurons showing intrinsic generation of 20- to 70-Hz repetitive firing, or chattering cells,38 were found to constitute 10-15% of intracellularly-recorded neurons, with each chattering cell diverging into a large population of cortical neurons. The repetitive firing by chattering cells was not spontaneous but was induced by suprathreshold depolarizing current injection,38 suggesting that gamma oscillations could be elicited by afferent stimulation of these cells in vivo.
The negative feedback interaction between pyramidal cells and fast-spiking interneurons could also generate gamma oscillations. For example, a characteristic phase relationship during gamma oscillation was observed between pyramidal cells and interneurons in the rodent hippocampus, such that interneurons fired a few milliseconds after pyramidal cells.28 Excitatory input from pyramidal cell firing likely induces inhibitory interneurons to generate synchronized activity, thus imposing gamma oscillation onto the entire local network. How-ever, the combined action of pyramidal cells and GABAergic interneurons does not suffice to explain long-range gamma synchronization. Most gamma oscillations occurred with zero-phase lag, indicating modulation of neuronal activity without any temporal delay between areas. This is intriguing, since many factors, such as conduction time and synaptic delay, contribute to delays in propagation of neural signals in cortical networks. Electrical gap junctions may be relevant to rapid oscillatory induction. Electrical gap junctions have been observed between interneurons and between pyramidal cells and interneurons, and blockade of gap junctions has been reported to reduce gamma oscillation (Figure 1).39
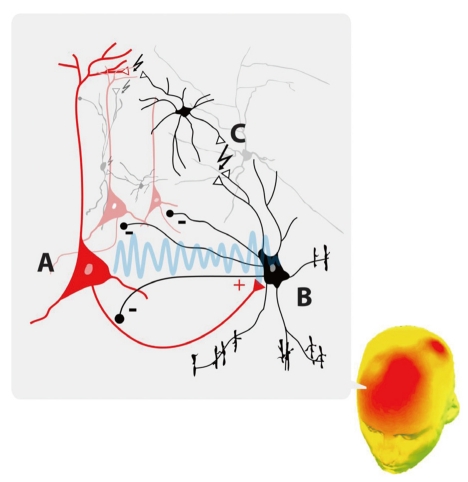
Schematic representation of the GABAergic interneurons and pyramidal neurons that generate gamma oscillation. A) Pyramidal neurons send excitatory signals to B) chandelier cells, a type of fast-spiking, parvalbumin-producing GABAergic interneuron, which in turn sends inhibitory signals back to the pyramidal cells. Activated interneurons can propagate inhibitory signals to multiple pyramidal cells and other electrically coupled (↯) interneurons via C) electrical gap junctions. The excitatory input from pyramidal cells and the inhibitory responses of the GABAergic interneurons generate synchronized activity imposing gamma oscillation onto the entire local network.
CELLULAR MECHANISMS FOR GAMMA-RANGE DEFICITS IN SCHIZOPHRENIA
Combined networks of pyramidal neurons and GABAergic interneurons are most likely the neural substrates that generate gamma oscillation. Abnormalities in both systems have been reported in patients with schizophrenia. For example, a post-mortem study reported that the volume of pyramidal neurons40 and the densities of axon terminal markers in the auditory cortex41 were lower in patients with schizophrenia than in normal controls. Patients with schizophrenia also showed deficits in glutamatergic synaptic connectivity in pyramidal cells.42 Phencyclidine an antagonist of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptor, has been shown to induce schizophrenia-like symptoms, including paranoid ideation, depersonalization and hallucinations, via hypofunctioning of NMDA receptors in pyramidal cells.43-45
Deficits in GABAergic interneurons have been found consistently in schizophrenia. A postmortem study found that the level of mRNA encoding the 67 kD isoform of glutamic acid decarboxylase (GAD), the key enzyme in GABA synthesis, was lower in the dorsolateral prefrontal cortex46 and the anterior cingulate cortex47 of patients with schizophrenia than in normal controls. Moreover, the level of mRNA encoding the neuronal GABA transporter-1 (GAT-1) protein in the prefrontal cortex48 and the density of GABA immunoreactive transporter axons termed "cartridges", which synapses exclusively at the axon initial segment of pyramidal cells in the dorsal prefrontal cortex,49 were lower in patients with schizophrenia than in controls. The GABAergic deficits in schizophrenia were mostly in the basket and chandelier types of interneurons,50 both of which have fast-spiking and parvalbumin-producing properties,43 which seem crucial for generating gamma oscillation.5,51 A study of mRNA transcripts encoding GABA-related substances in multiple cortical areas of patients with schizophrenia showed that levels of mRNA encoding somatostatin, parvalbumin, GAD, GABA A receptor, and GAT-1 were lower in patients with schizophrenia, with one of the greatest decreases observed for parvalbumin mRNA.52
Alterations in glutamatergic neurotransmission may lead to changes in the GABAergic system. In animals, treatment with an NMDA receptor antagonist induced reductions in GAD and parvalbumin mRNAs in GABAergic interneurons.53,54 NMDA hypofunction may lead to failure of exciting parvalbumin-producing GABA interneurons since these interneurons monitor NMDA receptor activity; thus hypofunctioning of NMDA may be falsely interpreted as inactivity of the pyramidal cell system, resulting in low production of GAD or GABA in the inhibitory interneuronal system.43 This hypothesis, that NMDA hypofunction is responsible for schizophrenia, also suggests deficits of gamma oscillation in individuals with schizophrenia.
GAMMA OSCILLATIONS IN SENSORY AND COGNITIVE FUNCTION
Sensory processing in healthy subjects and animals: Auditory steady state response
Auditory and visual stimuli at specific frequencies entrain EEG activity, called a steady state response (SSR), an evoked EEG potential whose frequency components remain constant in amplitude and phase during sensory processing of presented stimuli.55 In healthy subjects, amplitude modulated tones or click sounds evoked auditory SSR (ASSR), with stimuli of about 40 Hz inducing maximal spectral power.56 Although superposition of mid-latency event related potentials (ERPs) may generate these 40 Hz ASSRs,57,58 gamma range ASSRs reflect the phase reorganization of neuronal responses with their intrinsic resonance frequency, rather than the superposition of mid-latency ERPs.59 This superposition hypothesis could not explain the shortened phase delay of ASSR after repeated stimuli or continued phase synchronization after offset of a stimulus.60,61
Studies in animals have shown that the auditory cortex and the subcortical structure, including the hippocampus and inferior colliculus, play key roles in the generation of ASSRs.62-64 In cats, lesions in the lower auditory structures, including the inferior colliculus, were reported to decrease the phase synchrony of the ASSR in the gamma range.62 Ablation of the auditory cortices and inferior colliculi was reported to decrease ASSR to 40 Hz trains of clicks.64 Rats with neonatal ventral hippocampal lesions failed to show increased intensity and synchronization of ASSR after injection of GABA A receptor agonist, findings observed in control rats.65
Sensory processing in schizophrenia: ASSR
Deficits in ASSR have been reported in patients with schizophrenia.60 The spectral power on frontal EEG channels evoked by 40 Hz clinic trains was lower, whereas the phase delay, defined as the time gap between a click and the EEG peak response while listening, was higher in patients with schizophrenia than in control subjects.60 The decreased phase delay after stimulus offset observed in patients with schizophrenia compared with control subjects suggested that the neuronal assembly in patients with schizophrenia had deficits in the synchronization and/or desynchronization to presented stimuli.60 In addition, patients with schizophrenia showed reductions in evoked power and phase synchronization to steady state auditory stimulation in the gamma range.66 Moreover, relatives of patients with schizophrenia who had schizophrenic spectrum personality symptoms showed reduced gamma power on the frontal channels to steady state auditory stimulation at 40 Hz, although gamma power was not lower in patients with schizophrenia than in control subjects.67 In contrast, patients with schizophrenia taking atypical antipsychotics showed enhanced gamma power at 40 Hz stimulation compared with patients taking conventional antipsychotics.67
Sensory integration and object representation in healthy subjects and animals
Following the first report of gamma oscillations in hedgehog brains,13 gamma oscillations in human brains were first reported in intracranial recordings of patients with epilepsy while the patients performed simple visual tasks.68 However, the significance of gamma oscillations was not known until they were shown to play a role in integrating sensory information.24,69,70 Local field potential (LFP) and multiunit activity (MUA) were measured in the primary visual cortices of cats responding to visual stimuli.24 LFP measures the synchronized neural activity of local populations of neurons, whereas MUA measures spikes of several neurons. Therefore, simultaneous recording of LFP and MUA can reveal the relationship between cortical oscillations and individual neuronal activity. Individual neuronal firings, as measured by MUA, were found to be synchronized to the rhythm of gamma oscillations of LFP if the neurons were coding common primary visual properties of a visual stimulus, even if the neurons were spatially discrete.24 Similarly, recording the neuronal activity in two areas of cat visual cortices, the posteromedial lateral suprasylvian area and area 17, which function in global pattern and fine grain analyses of visual objects, respectively, showed that the two groups of neurons fired synchronously on gamma oscillation in response to coherently moving lines, but not to two lines moving in opposite directions.70 These findings suggested that two separate groups of neurons integrated information across different feature domains through temporal synchronization on gamma oscillation.
Increased gamma oscillations were observed in patients requiring sensory integration, from simple perceptions like seeing a moving bar to higher cognitive processes including object representation. The perception of illusory objects induced gamma oscillations in human brains. The spectral power of gamma oscillations in the occipital area of scalp EEG was fo-und to be higher for coherently downward moving lines than for irregularly moving lines.71 Healthy subjects watching coherent but not incoherent dot motion showed increased gamma activity around 40 Hz (Figure 2).72

Increase in gamma activity during the perception of coherent and incoherent dot motions by healthy subjects. The left column shows the two stimulus conditions, for coherently displaced dots from left to right (upper) and for incoherently displaced dots at randomly generated angles (lower). The right column shows time-frequency spectrograms of averaged electroencephalography power for coherent (upper) and incoherent (lower) dot motions in the channel Pz (Figure courtesy of Giri P. Krishnan).
Perceiving the Kanizsa triangle also induced gamma oscillations, whereas non-triangle control stimuli did not.73,74 Similarly, illusory triangles increased spectral activity at the 70 Hz MEG frequency band compared with no triangles and increased activity at 90 Hz compared with real triangles.75 Recognition of illusory triangles requires the identification of all elements comprising the triangle and their linkage globally across space.76 Therefore, the emergence of gamma oscillations following the recognition of illusory objects suggests that gamma oscillations play a key role in binding information for object representation in the brain.
Sensory integration and object representation in schizophrenia
In time frequency analysis of ERP, the phase-locking factor (PLF) measures the consistency of the phases of EEG responses across trials of presented stimuli at each frequency. PLF that measures the synchronization of a neuronal assembly to a stimulus may be a better biomarker than measures of EEG power for patients with schizophrenia spectrum disorders.77 Patients with schizophrenia showed deficits in PLF while perceiving both illusory and non-illusory stimuli, with deficits more prominent for illusory than non-illusory stimuli.78 As the PLF gap between illusory and non-illusory stimuli became larger in control subjects, so did the ability to recognize illusory stimuli, as measured by the difference in reaction time (RT) between illusory and non-illusory stimuli. In patients with schizophrenia, however, there was no such relationship between PLF and performance.78 Although gamma synchronization during recognition of Mooney faces was lower in patients with schizophrenia than in control subjects, the gamma power was not decreased during Mooney face recognition in patients with schizophrenia.79
Working memory and attention in healthy subjects and animals
Scalp EEGs showed that gamma activities were increased in subjects asked to find a hidden dog figure in a picture with scattered black blobs, while keeping the dog figure in mind.80 Increases in gamma activity were observed when subjects were asked to match a previously presented figure, but not in the control task requiring no memorization.18 The involvement of gamma oscillation in memory tasks was also reported in macaque monkeys, with elevated coherence in gamma frequency between a single neuronal activity and LFP in the lateral intraparietal area of the brain during the memory period.19
Attention increased gamma oscillations in the brains of animals and human subjects. When macaque monkeys attended to visual stimuli, their activated neurons showed increased gamma oscillation,15 with selective attention to visual stimuli increased gamma oscillation of LFP in area V4.14 In monkeys trained to respond to a stimulus, the RT was decreased if the degree of synchronization was increased between gamma oscillation of LFP and individual neuronal firings during the time period before response cues.14 Gamma oscillation was also increased by attention in human subjects. Scalp EEG showed that gamma activity was higher for attended stimuli over parieto-occipital areas.81 Only consciously perceived stimuli were found to induce long-range gamma phase synchrony, whereas subliminal stimuli increased local gamma oscillation.23 However, attention is not mandatory in generating gamma oscillation considering early studies using the visual systems of anesthetized animals to generate gamma oscillations.24,69,70
Working memory and attention in schizophrenia
Patients with schizophrenia showed a deficit in gamma activity in the frontal area during mental arithmetic tasks.82 Gamma activity during the retrieval period of working memory was reduced in patients with schizophrenia compared with healthy subjects.83 Healthy subjects had increased gamma activity in the prefrontal cortex in response to increased demands of executive control in a working memory task, a finding not observed in patients with schizophrenia.84 Similarly, healthy subjects, but not patients with schizophrenia, showed increased gamma activity with increased working memory load.85 Patients with first onset schizophrenia were recently found to show reduced gamma power in the frontal area during the delay period of a preparatory cognitive task compared with control subjects, regardless of medication status.86
The evoked gamma oscillation studies using the auditory oddball task have shown inconsistent results in patients with schizophrenia. Gamma activity, narrowly defined as 37-41 Hz in response to standard tones of auditory oddball tasks, was lower in medicated patients with schizophrenia than in control subjects.87 In addition, gamma activity to target tones was also lower 200-400 ms after a stimulus in patients with schizophrenia than in control subjects.87 However, evoked gamma activity to standard tones of the auditory oddball task did not differ between unmedicated patients with schizophrenia and control subjects, whereas gamma activity in response to target tones was lower in patients with schizophrenia than in controls.88 Abnormal gamma activity was also observed in the phase coherence between EEG channels in schizophrenia. For example, impaired fronto-central gamma coherence was reported in unmedicated patients with schizophrenia,89 and decreased evoked gamma power in auditory oddball paradigms was observed in medicated first-episode patients with schizophrenia.90 However, evoked gamma activity in the auditory oddball task was reported similar in control subjects and chronic patients with schizophrenia.91 A study of a modified auditory oddball task in a large number of patients with schizophrenia found that early-evoked gamma activity was lower in these patients than in a control group.92
In contrast to conflicting results on evoked gamma power in schizophrenia, gamma phase locking was consistently reported to be lower in schizophrenia during visual91 and auditory oddball tasks.66,93 In a study using ASSR to evaluate PLF over a broad range of stimulus frequencies, control subjects showed pronounced increases in PLF and power around the gamma frequency range of stimuli, whereas patients with schizophrenia showed deficits in both PLF and mean power in broadband frequencies, including gamma frequency. A noise embedded in the 40 Hz stimulus decreased PLF only in control subjects, whereas patients with schizophrenia showed diminished overall PLF.94
GAMMA OSCILLATION AND SYMPTOMS OF SCHIZOPHRENIA
Interestingly, symptoms of schizophrenia have been reported to correlate with increased synchronization of gamma oscillation, although mean gamma synchronicity was lower in patients with schizophrenia than in control subjects.79,95-97 Phase-locking effects in the occipital area, as measured by differences in PLF during the perception of Gestalt stimuli, correlated with scores of positive symptoms.96 The phase-locking effects in the parietal area correlated with negative symptoms in patients with schizophrenia.96 Increased gamma phase synchrony averaged across all channels during a Gestalt perception task was positively correlated with positive symptoms, such as delusions and hallucination.79 Using ASSR, PLF of the 40 Hz harmonic of the 20 Hz stimuli was reported to correlate with positive symptoms in the frontocentral areas of patients with schizophrenia.97 Increased gamma synchrony may reflect the retrieval of stored experiences due to the role of gamma activity in internal representations.98
CONCLUSION
Gamma oscillation is a universal phenomenon, found throughout all areas of the brain and across species, from simple perception to higher cognitive functions. The dominant frequency of gamma oscillation changes continuously according according to cortical locations from moment to moment.99 Different neuronal groups may communicate during the time window of gamma oscillation,100 and the precise phase of gamma oscillations may determine whether neuronal activity could be effectively transmitted among cortical areas.101 Therefore, gamma oscillation may be a temporal limitation in the functional connectivity of human and animal brains, modulating efficient information processing at macro and micro levels of neural circuits. The inhibitory feedback of GABAergic interneurons combined with activations of pyramidal neurons seems to generate gamma oscillation. Abnormalities of the neuronal system, including pyramidal neurons and GABAergic interneurons, could result in the deteriorated gamma oscillation observed in schizophrenia. Deficits of gamma oscillation and impaired neuronal communication would result in erroneous processes in a variety of both basic and higher cognitive functions, including sensory perception, coherent feature binding, attention, memory and object representation, all of which would lead to the positive and negative symptoms of schizophrenia.