Bone Density in Chronic Schizophrenia with Long-Term Antipsychotic Treatment: Preliminary Study
Article information
Abstract
Objective
Decreased bone mineral density has been found in the chronic schizophrenic patients who have been given a long-term administration of antipsychotics. Hyperprolactinemia from the antipsychotics and the negative symptom of schizophrenia were considered as the causes for this finding. In this study, the effect of hyperprolactinemia and the negative symptom of schizophrenia on bone mineral density was investigated on male schizophrenic patients.
Methods
The cross-sectional study was carried out with the subjects of 45 male schizophrenic patients who have undertaken the monotherapy with risperidone, olanzapine and clozapine for at least one year. The demographic factors, clinical symtoms, bone mineral density and hematological test were examined for all the subjects.
Results
No significant relationship was found between hyperprolactinemia and the decreased bone mineral density in the subjects. The negative schizophrenia symptom of the subjects showed a significant effect on the decreased bone mineral density.
Conclusion
The decreased bone mineral density finding in the male schizophrenic patients may be caused by the negative schizophrenia symptom rather than the hyperprolactinemia due to the antipsychotics. Additional studies are further required regarding other factors that may affect the decreased bone mineral density such as activity, calcium intake and exposure to sunlight.
INTRODUCTION
Osteoporosis is a disease that happens when the bone matrix, which is the base of bone growth, is produced insufficiently or when calcium decreases considerably in bone and, as a result, bone mineral density decreases.1 Bone mineral density is affected by the peak bone mineral density predetermined genetically, but it is also affected by various other factors. That is, it is lower in women, those in their puberty, those whose menarche has been delayed and those with premature menopause, and it is influenced by insufficient calcium intake, lack of exercise, excessive smoking, alcohol or caffeine abuse, etc.2-4 In the United States, Osteoporosis increases the risk of bone fracture, and the incidence is increasing every year, as there were 1,300,000 cases in 1990 but there are expected to be 2,600,000 cases in 2025.5,6 Patients with schizophrenia have many risk factors of osteoporosis because of their inadequate eating habits, low activity, polydipsia, excessive smoking, alcohol abuse, etc.7 In addition, the risk of bone fracture can be heightened both by orthostatic hypotension caused by drugs and by falls caused by dizziness.8
There have been several reports that the decrease of bone mineral density is observed in patients with chronic schizophrenia.9-11 Early research suggested that such a decrease might be related to polydipsia or serum calcium concentration, but it could produce no significant correlation with the decrease of bone mineral density.12,13 On the contrary, osteoporosis or osteopenia was observed in around 80% of the patients with a prolactin-secreting tumor, and the change of sex hormones caused by hyperprolactinemia was believed to be related to the decrease of bone mineral density.14,15 This suggests that hyperprolactinemia and the following change of sex hormones observed in patients with chronic schizophrenia may be related to the decrease of bone mineral density.
There have been many reports that hyperprolactinemia happens in patients with chronic schizophrenia who have taken typical antipsychotics and risperidone for a long period in the past, and the decrease of bone mineral density is observed in such patients.16-18 The incidence was greater among the female menopausal patients who underwent a drastic change in sex hormones. On the other hand, there were also reports that there was no significant correlation between the prolactin concentration and bone mineral density in a group of patients who used typical antipsychotics for a long time, and the factors causing the decrease of bone mineral density in such patients were lack of calcium intake, eating disorder, lack of sunlight exposure and insufficient exercise due to the negative symptom rather than the effect of prolactin.19,20 As such, it is quite controversial to claim whether the decrease of bone mineral density observed in schizophrenic patients is caused by antipsychotics.Thus, this study was performed in order to determine whether the decrease of bone mineral density observed in male schizophrenic patients who have taken antipsychotics for a long period, and are less influenced by a change of sex hormones as in menstruation or menopause, is caused by hyperprolactinemia resulting from the antipsychotics or by negative symptoms that may result in the risk factors of osteoporosis such as lack of calcium intake, eating disorder, lack of sunlight exposure or lack of exercise.
METHODS
Subjects
This study was carried out with 20-year-old or older patients who were under treatment as outpatients or inpatients at the Psychiatric Department of Seoul Veterans Hospital from March 1, 2007 to June 30, 2007. The patients were limited to the men who had been diagnosed with schizophrenia through the review of medical records by DSM-IV and clinical interviews by a psychiatrist and a resident, and who had used only one drug among risperidone, olanzapine or clozapine for the previous one year or longer.
Criteria for exclusion are as follows: 1) those with a history of alcohol or other substance dependence or abuse; 2) those with a serious internal disease that may affect bone mineral density; 3) those with a history of bone fracture within one year; 4) those with diseases or symptoms that can impair physical mobility, including Parkinson's disease and extrapyramidal side effects; 5) those with another accompanying disease in Axis I according to DSM-IV; and 6) those who were taking a mood stabilizer, antidepressant or anxiolytic that can affect bone mineral density in addition to the antipsychotic. Each of the subjects provided a written consent for their participation in the present study after being informed about the objective of this study.
Methods
The clinical information of all the subjects, including the demographic information and drug treatment history, were collected by the medical records review and physical examination and their clinical symtoms were assessed based on the Positive and Negative Symptoms Scales (PANSS). The antipsychotic doses of risperidone 1 mg, olanzapine 2.5 mg and clozapine 50 mg were converted to chlorpromazine 100 mg, which is an equivalent dose.
The bone mineral density T-score and Z-score of the subject group were measured with dual-energy X-ray absorptiometry (DEXA; hologic discovery W). The bone mineral density was measured at the lumbar spine L1-L4, the femoral neck, the trochanteric regions of the left hip and the intertrochanteric regions of the left hip.
Blood samples were taken from all the subjects at 8 o'clock in the morning at the Department of Clinical Laboratory in the hospital, and using the samples, we measured hormones that may affect bone metabolism including serum estradiol, progesterone, testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid hormones [ex. triidothyronine (T3), free thyroxine (fT4), thyroid stimulating hormone (TSH)], prolactin and cortisol. In addition, bone markers such as plasma bone alkaline phosphatase, osteocalcin, C-terminal telopeptide type I collagen (ICTP), urinary calcium and phosphorus were measured. The study protocol was approved by the institutional review board at Seoul Veterans Hospital.
Statistical analysis
A one-way analysis of variance was performed in order to compare demographical characteristics, the mean values of the test results such as PANSS, Body Mass Index (BMI) and blood prolactin concentration, with that of the bone mineral density level among the subject groups. Pearson's rank correlation analysis was performed on all the patients to determine the correlations among the parameters, and based on these results, a multivariate regression analysis was performed with the variables that have significant correlations with each corresponding bone and identify the factors that significantly affect bone mineral density.
In addition, a chi-square test was performed to see whether osteoporosis or osteopenia are more frequently examined in a specific drug. The data were analyzed using SAS Version 8.1, and the statistical significance was determined based on the significance level of p<0.05.
RESULTS
Comparision of the patients' demographical and clinical characteristics
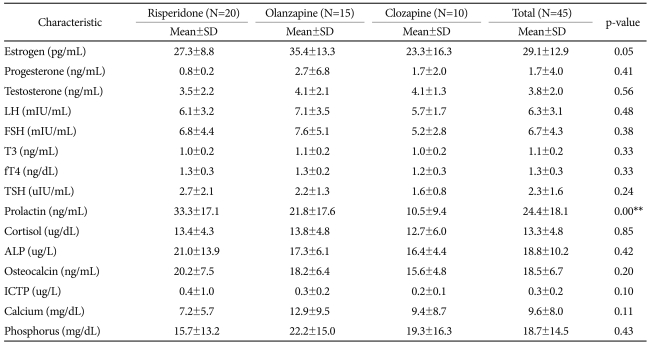
A total of 45 patients, including 20 in the risperidone group, 15 in the olanzapine group and 10 in the clozapine group, participated in this study. All of them completed the study. There was no significant difference among the three groups in average age, disease duration, the chlorpromazine equivalent dosage of antipsychotic and the positive sub-scale and negative sub-scale of PANSS (Table 1).
Comparison of the laboratory and radiologic examination results in each patient group
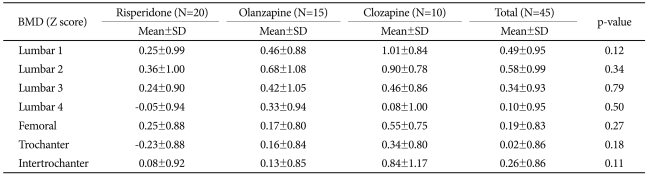
In the laboratory test performed for each patient group, no significant difference was found in serum estrogen, progesterone, testosterone, FSH, LH, T3, fT4, TSH, cortisol, bone, alkaline phosphatase, osteocalcin, ICTP, urinary calcium and phosphate. The serum prolactin concentration of the risperidone group was significantly higher than that of the clozapine group (Table 2). In the results of radiologic examination, bone mineral density Z-score was not significantly different among the subject groups (Table 3). In the risperidone group, 15 out of the 20 subjects had hyperprolactinemia, in addition to 3 subjects with osteoporosis and 3 subjects with osteopenia. In the olanzapine group, 6 among the 15 subjects had hyperprolactinemia, and 7 subjects had osteopenia. Among the 10 subjects in the clozapine group, 1 subject had hyperprolactinemia and 1 subject had osteopenia. We analyzed whether a specific drug is more related to osteopenia or osteoporosis, but no significant difference was observed among the 3 drugs.
Correlation and regression analysis among demographic variables, laboratory variables and bone mineral density
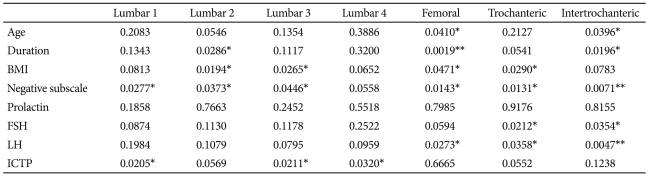
We analyzed the correlation between the major variables that are likely to affect bone metabolism, including bone markers and bone mineral density Z-score (Table 4). With respect to the correlations with bone mineral density, the average age was in a negative correlation with bone mineral density in the femoral neck, the trochanteric regions of the left hip and the intertrochanteric regions of the left hip; the disease duration was in a negative correlation with bone mineral density in the lumbar spine L2, the femoral neck, the trochanteric regions of the left hip and the intertrochanteric regions of the left hip; the BMI was in a positive correlation with bone mineral density in the lumbar spine L3, the femoral neck and the trochanteric regions of the left hip; and the sub-scale of negative symptoms was in a nega tive correlation with bone mineral density in the lumbar spine L1-3, the femoral neck, the trochanteric regions of the left hip and the intertrochanteric regions of left hip. In addition, the FSH was in a negative correlation with bone mineral density in the trochanteric regions of the left hip and the trochanteric regions of the left hip; the LH was in a negative correlation with bone mineral density in the femoral neck, the trochanteric regions of left hip and the intertrochanteric regions of left hip; and the C-terminal telopeptide was in a negative correlation with the lumbar spine L1, 3 and 4. Besides, no significant correlation was observed between other laboratory findings and bone mineral density. The correlation between hyperprolactinemia and bone mineral density was analyzed only with the 22 patients with hyperprolactinemia over all the subject groups, but no significant correlation was found. Based on these results, a multivariate regression analysis was performed with the variables that have significant correlations with each corresponding bone including age, disease duration and BMI of the subjects. The results showed that bone mineral density in the lumbar spine L1 was significantly affected by the sub-scale of negative symptoms (p=0.02) and C-terminal telopeptide (p=0.02); in the lumbar spine L2 by disease duration (p=0.02) and the sub-scale of negative symptoms (p=0.03); in the lumbar spine L3 by C-terminal telopeptide (p=0.02); in the lumbar spine L4 by C-terminal telopeptide (p=0.03); in the femoral neck by the average age (0.04) and disease duration (p=0.00); in the trochanteric regions of the left hip by the sub-scale of negative symptoms (p=0.02); and in the trochanteric regions of the left hip by the sub-scale of negative symptoms (p=0.03).
DISCUSSION
Typical antipsychotics or risperidone blocks the dopamine D2 receptor of lactotrophs in the anterior pituitary and the prolactin secretion inhibiting function, and consequently, causes hyperprolactinemia.21,22 Hyperprolactinemia directly inhibits osteoblast or hinders the action of LH or FSH, and thus causes a decrease of estrogen concentration.23,24
Women's bone mineral density is highest during the period from the early 30s to the mid 30s, and then decreases slowly, and the decrease is accelerated for 3 to 5 years from the beginning of menopause, and after that, it decreases by 1.0 to 1.5% a year.25,26 Menopause accelerates bone loss due to the effect of decrease in sex hormones. Estrogen decreases bone loss by inhibiting the function of osteoclast, and progesterone accelerates bone formation by stimulating osteoblast.27,28 Thus, the decrease of sex hormones by hyperprolactinemia accelerates the decrease of bone mineral density.
Patients with chronic schizophrenia actually show a very high prevalence of osteoporosis and bone fracture compared to normal people, and there have been many reports that bone mineral density decreased markedly in them.29 Hence, there have been many studies that presumed a correlation between the decrease of bone mineral density found in patients with chronic schizophrenia and hyperprolactinemia caused by long-term medication with antipsychotics.
In a study of chronic schizophrenic patients whose average age was 43, Abraham et al.30 reported a significant negative correlation between antipsychotic-induced hyperprolactinemia and bone mineral density and explained that this mechanism is the same as that of the bone mass decrease found in patients with prolactin-secreting adenomas. In addition, Naidoo et al.31 conducted a short survey on the potential impact of antipsychotics agents and reported that elevated rates of osteoporosis and pathological fractures were observed in schizophrenic patients treated with antipsychotics for a long period, and they might have been caused primarily by hyperprolactinemia induced by antipsychotics and secondarily by the lower estrogen and testosterone levels.
However, such a mechanism may not be applied to all the cases since the subjects of many studies in the past were female schizophrenic patients and the effect of sex hormones on the decrease of bone mineral density is different in male schizophrenia patients.
Meanwhile, there have also been reports that the decrease of bone mineral density observed in chronic schizophrenic patients medicated with antipsychotics for a long-term period is caused by the negative symptoms of schizophrenia itself rather than by the drugs. Abraham et al.32 reported that higher rates of bone formation and resorption were observed in the schizophrenic patients with decreased bone mineral density, but there was no significant correlation between serum prolactin and bone mineral density. Rather, they reported that the common negative symptoms in chronic schizophrenic patients were related to the decrease of bone mineral density. In addition, Hummer et al.33 reported that the decrease of bone mineral density observed in patients with chronic schizophrenia was not correlated with hyperprolactinemia, and it was more reasonable to consider it as a result of the patients' negative symptoms.
In this study, the sex hormone concentration of all the patients fell within the normal range and no significant correlation was found between sex hormones and the decrease of bone mineral density or between prolactin and the decrease of bone mineral density. These results are different from the previous studies, which concluded that hyperprolactinemia observed in schizophrenic patients may directly cause a decrease of bone mineral density or that the reduction of sex hormone levels induced by hyperprolactinemia may cause a decrease of bone mineral density, suggesting that the pathophysiology of the decreased bone mineral density found in the male schizophrenic patients may be different from that of females.
Generally, men have a higher level of peak bone density than women, and this prevents the exacerbation of osteoporosis.34 In addition, both testosterone and estrogen act as resistance factors for the decrease of bone density; however, while the concentration of estrogen in the blood of women rapidly drops after menopause, the concentration of testosterone in the blood of men declines gradually.35 Young Caucasian women appear to be particularly vulnerable to developing hyperprolactinaemia and the associated hypogonadism and bone loss. The occurrence of menstrual dysfunction should alert clinical suspicions of hyperprolactinaemia and bone de-mineralisation.36 Meanwhile, the age of onset of schizophrenia for men is generally lower than for women. In other words, at the same age range, male patients with schizophrenia could have longer periods to contract the disease in comparison to female patients with schizophrenia. Moreover, early onset of the disease can influence the peak bone density.37 Since there are too many factors involved in the decrease of bone density in pathophysiology that relate to the differences between men and women, it is extremely difficult to discern contributions of individual factors. Accordingly, a future study is required on whether the decrease of bone density among male schizophrenics occurs due to different mechanisms in comparison to female patients by controlling the factors.
On the other hand, when significant factors affecting the decrease of bone mineral density in the subject groups were investigated by including the factors which are known to cause a decrease of bone mineral density, it was found that the negative symptoms scale of PANSS and the blood concentration of ICTP have a significant effect on the decrease of bone mineral density. In addition, the risperidone group showed a significantly higher blood prolactin level than the olanzapine group and the clozapine group, but the rate of observed osteomalacia or osteoporosis was not significantly different among the 3 groups. These results support the hypothesis that the decrease of bone mineral density observed in schizophrenic patients medicated with antipsychotics for a long period could be caused more by the negative symptoms of schizophrenia than by the drugs.
Among the causes of the decrease of bone mineral density, factors that can be correlated with the negative symptoms of PANSS include lack of load-bearing exercise due to a decrease in activities, deficiency in Vitamin D metabolism due to lack of sunlight exposure and lack of calcium intake due to eating problems.38,39
Vitamin D can be taken partially from food, but it is mostly generated as provitamin D in the body reacts to ultraviolet rays on the skin's surface and is converted to vitamin D.40 For the generation of vitamin D, indoor sunlight transmitted through the window is insufficient, and the outdoor ultraviolet wavelength is important.41 In addition, because bone cells grow as they are stimulated by the pressure of gravity, light exercise is important to produce such an effect.42
However, chronic schizophrenic patients with severe negative symptoms cannot have sufficient outdoor activities, and this results in the lack of load-bearing exercise. Therefore, in combination with lack of calcium intake, factors such as lack of exposure to ultraviolet rays and lack of load-bearing exercise lower the growth of bone cells and increase bone reabsorption, and thus cause the decrease of bone mineral density.
However, the risk factors can be caused by positive symptoms, including delusion or hallucination of patients with schizophrenia. Moreover, a study on whether inherent pathophysiology of negative symptoms directly affects the decrease of bone density is still lacking. Against this backdrop, we recommend an even more controlled study on whether the decrease of bone density is incurred by risk factors caused by negative symptoms of patients with schizophrenia or by another pathophysiology. Serum bone specific alkaline phosphatase and osteocalcin, the bone formation markers, were within the normal range in all of the subject groups, and they did not show a significant correlation with bone mineral density. However, bone absorption marker, c-terminal telopeptides type I collagen, increased in the subject groups and had a significant effect on the decrease of bone mineral density in the lumbar spine L1, 3 and 4.
Continuous bone turnover takes place in the skeleton as old bone is removed by bone absorption and new bone is generated by bone formation. However, while it takes at least 3 to 4 years to determine the change of bone mineral density through radiologic examination, biochemical bone markers allow more sensitive tests through which the change of bone metabolism can be assessed in a relatively short time.43 Various biochemical bone markers have been known, but DPD, NTX and CTX are most recommended as bone adsorption markers, and BSALP and OC as bone formation markers.44 In this study, the increase of ICTP had a significant effect on the decrease of bone mineral density in the lumbar spine L1, 3 and 4, whereas other bone markers were not correlated with the decrease of bone mineral density in the patient groups. ICTP has a low specificity in reflecting bone absorption, since it can be generated by the decomposition of not only bone but also other collagen.45,46 Thus, it should be understood that the decrease of bone mineral density is correlated more with physical changes causing the increase of ICTP than with the increase of ICTP itself.
The limitations of this study are as follows: First, the subjects of this study were limited to male patients. In previous studies, the decrease of bone mineral density was more frequently found in female schizophrenic patients than in male schizophrenic patients, which suggests that sex hormones play an important role in the decrease of bone mineral density. Thus, the results of this study should be carefully interpreted. Second, because sunlight exposure, amount of physical exercise and oral calcium intake were not controlled by the subject groups, it was not verified whether the negative symptoms found in the subject groups are directly correlated with such factors or not. Third, there might have been a difference in the amount of activities depending on the environment of the subject groups that included inpatients and outpatients, but such a factor was not controlled. Fourth, there was no comparison of the result to a control group of schizophrenic patients who did not take antipsychotics.
This study investigated the decrease of bone mineral density in male schizophrenic patients who had taken one drug among risperidone, olanzapine or clozapine for a long period of time. The results showed that the prolactin concentration did not have a significant effect on bone mineral density in the patient groups, and the negative sub-scale of PANSS had a significant effect on the decrease of bone mineral density. Therefore, follow-up research with suchlike patient groups needs to be conducted while controlling factors such as negative symptoms, exposure to sunlight, calcium intake and amount of excercise.