Effects of Psychotropic Drugs on Seizure Threshold during Electroconvulsive Therapy
Article information
Abstract
Objective
To analyze the relationship between seizure threshold (ST) and psychotropic drugs in patients treated with ECT.
Methods
We examined clinical data from 43 patients. ST was titrated at each treatment session. We examined associations between ST and psychotropic drugs using multivariate correlation analyses. Data are presented as initial ST, the difference in ST between the first and 10th sessions (ΔST10th), and the mean difference in ST between the first and last sessions (mean ΔSTlast).
Results
Multivariate regression analyses showed associations between initial ST and the total chlorpromazine-equivalent dose of antipsychotics (β=0.363, p<0.05). The total fluoxetine-equivalent dose of antidepressants was associated with ΔST10th (β=0.486, p<0.01) and mean ΔSTlast (β=0.472, p<0.01).
Conclusion
Our study elucidated possible effects of psychotropic drugs on ST shifts. Larger doses of antipsychotics were associated with higher initial ST, whereas higher doses of antidepressants were associated with stronger shifts in ST.
INTRODUCTION
Various kinds of brain stimulation therapies are widely used to treat discrete psychiatric disorders. Deep brain stimulation, repetitive transcranial magnetic stimulation, and vagus nerve stimulation are just a few of the tools used to stimulate the brain's neurons in the hope of achieving beneficial effects for patients with psychiatric diseases. Electroconvulsive therapy (ECT) is one of the oldest and most popular ways to achieve therapeutic effects by stimulating the brain with the help of electrical currents.12 ECT had a poor initial reputation due to the use of electricity directly on the patient's head. But treatment procedures have evolved significantly and proven to be safe and effective. The introduction of anesthesia and muscle relaxants greatly improved the subjective treatment experience, in addition to decreasing serious side effects such as spine fractures or dental issues. The therapeutic effect of ECT emerges from the induction of a generalized seizure.3 The minimal amount of electrical energy needed to induce seizures is known as the seizure threshold (ST). It is commonly believed that treatment efficacy is related to stimulus dose relative to ST, but higher stimuli usually also increase unwanted side effects.3456 Many treatment protocols, therefore, advise the use of just-above-threshold energy levels and provide guidance to find those numbers.78 Clinicians can use different algorithms to estimate initial ST, though some studies still emphasize the efficacy of empirical titration or fixed high initial dose methods.79
ST varies greatly by individual and can be affected by many factors.1 Previous studies agree that male sex, older age, higher body mass index (BMI), and bilateral electrode placement are associated with higher ST.2310 The contribution of benzodiazepines and anticonvulsant drugs to ST was partially confirmed by a Japanese study that found that prescriptions of benzodiazepines correlated with higher ST.11 Many efforts were made to counteract such increases in ST. The electrical waveform was changed from a sine wave to a brief pulse, further evolving to an ultra-brief pulse.4 Hyperventilation, adjustment of electrodes, and even proconvulsants were also considered, but not all trials were successful.1213 Despite such efforts, ways to predict ST remain understudied. Among the few studies that do exist, an even smaller number focus on factors that affect the shift of ST during the course of ECT. One study in Bangkok showed that the magnitude of ST shifts associates positively with the number of sessions and negatively to initial ST, change in seizure duration, and response status.14 Other studies pointed out that younger age and the use of psychotropic drugs may be associated with lower seizure thresholds, but none examined the effects of drugs on the shift of ST during ECT, even though most patients routinely take concurrent psychotropic medication while undergoing treatment.151617 Therefore, in this study, we focus on the effects of psychotropic drugs, both antipsychotics and antidepressants, on the ST shift in patients receiving ECT.
METHODS
Participants
We performed a retrospective study to investigate the effects of psychotropic drugs on ST among patients who received ECT at Korea University Guro Hospital (KUGH) between February 2009 and June 2015. Patients with a history of seizure disorders or other medical emergent conditions were excluded. A total of fifty-eight subjects received ECT during the study period. Patients were excluded if treatment was aborted due to side effects or any other reasons before the 10th session (n=12) because we intended to investigate the ST shift during the course of consecutive ECT sessions. We included 43 subjects in the final data analysis. Among them, 5 had hypertension, 7 had diabetes, and 3 patients had subclinical hypothyroidism as medical comorbidities. Patients' psychiatric disorders were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-R) by at least two experienced psychiatrists in the usual clinical settings at KUGH. ECT was administrated with concurrent antipsychotics and antidepressants. Benzodiazepine, mood stabilizers with anticonvulsant effects such as lithium and valproic acid, and z-drugs such as zolpidem were tapered out before initiating ECT and not used until all treatment sessions ended. We documented ECT variables (including the time interval between the last and current titration sessions) and concomitant medication using medical records. The study protocol was approved by the Institutional Review Board of KUGH, and we obtained written informed consent from all participants for use of their clinical data. Consent was taken from guardians if the participant was thought to be unable to make clear decisions himself.
ECT procedures
All patients were admitted to KUGH at least one week before starting ECT procedures. We used a MECTA SpECTrum 5000Q apparatus for seizure induction. Patients were hyperventilated with 100% oxygen for 10 seconds and anaesthetized using propofol (1–2 mg/kg). We used succinylcholine (0.5 mg/kg) to induce muscle relaxation and conducted clinical monitoring, including electrocardiographic and electroencephalographic (EEG), throughout the whole procedure. Propofol and succinylcholine doses were kept consistent throughout all treatment sessions. We placed ECT electrodes at the bilateral temporal areas and applied the electrical stimulus as soon as the patient reached the deepest stage of anesthesia. Initial stimulus strength varied between 10J and 15J, converted from the recommended initial 50 mC at 220 ohm patient impedance.8 Seizure induction was considered successful if the seizure lasted for more than 25 seconds. Stimulus strength was maintained after a successful session. After an unsuccessful trial, we increased stimulus strength stepwise by 2J to 5J per trial. We performed a maximum of 4 stimulations at each treatment session, with an interval of at least 30s between stimuli. Each patient underwent 10 to 20 sessions, based on the psychiatrist's clinical decision. We considered the stimulus strength of the first successfully induced seizure to be the minimal ST. We used the cuff method to determine seizure duration, along with mastoid EEG channels.
Statistical analysis
We present the following data: initial ST is the amount of electrical energy used to induce a successful seizure at the first session; ΔST10th is the difference in ST between the first and 10th sessions; mean ΔSTlast is the mean difference in ST between the first and last sessions. We used stepwise multivariate regression analyses to find associations between those data and the doses of psychotropic medication, adjusting for age, sex, and BMI. We performed univariate regression analyses to find associations between laboratory test results and ST. We used covariance analyses to find differences in variables between clinical subgroups divided by types of prescribed medication, adjusting for age, sex, and BMI. All statistical analyses were performed using the SPSS 19 statistical package (IBM Corp., Armonk, NY, USA).
RESULTS
Demographics and clinical variables
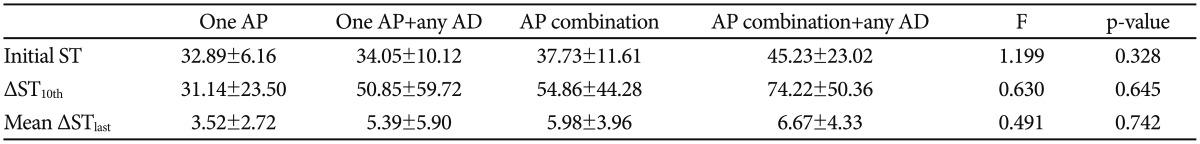
Of the 43 patients included in the study, 20 were male, and the other 23 were female. The mean age of all participants was 41.44 years (SD=15.89), with a total range from 15 to 71 years old. 29 patients were diagnosed with schizophrenia, 4 with schizoaffective disorder, 9 with major depressive disorder, and 1 with bipolar disorder, according to DSM-IV-R criteria. All medication was prescribed by psychiatrists. Use of benzodiazepines, z-drugs, and anticonvulsants was avoided during ECT administration. Patients were taking the following antipsychotics and antidepressants: clozapine (n=6), amisulpride (n=9), aripiprazole (n=5), olanzapine (n=18), risperidone (n=1), quetiapine (n=20), haloperidol (n=1), paliperidone (n=5), chlorpromazine (n=1), blonanserin (n=1), escitalopram (n=7), sertraline (n=1), mirtazapine (n=2), duloxetine (n=1), venlafaxine (n=3), amitriptyline (n=1), trazodone (n=1), bupropion (n=1). Participants took an average of 1.91 (SD=1.02, range 0–5) different psychotropic drugs during ECT. The mean number of types of antipsychotics and antidepressants used were 1.53 (SD=0.74, range 0–3) and 0.37 (SD=0.76, range 0–4), respectively. We converted the dose and types of antipsychotics and antidepressants into chlorpromazine (CPZ) and fluoxetine (FXT) equivalences, respectively.181920 For detailed information on drug dose equivalences (Table 1). Patients received an average of 14.4 sessions of ECT (SD=3.06, range 10–20). Sociodemographic data are summarized in Table 2.
Effect of psychotropic drugs on ST
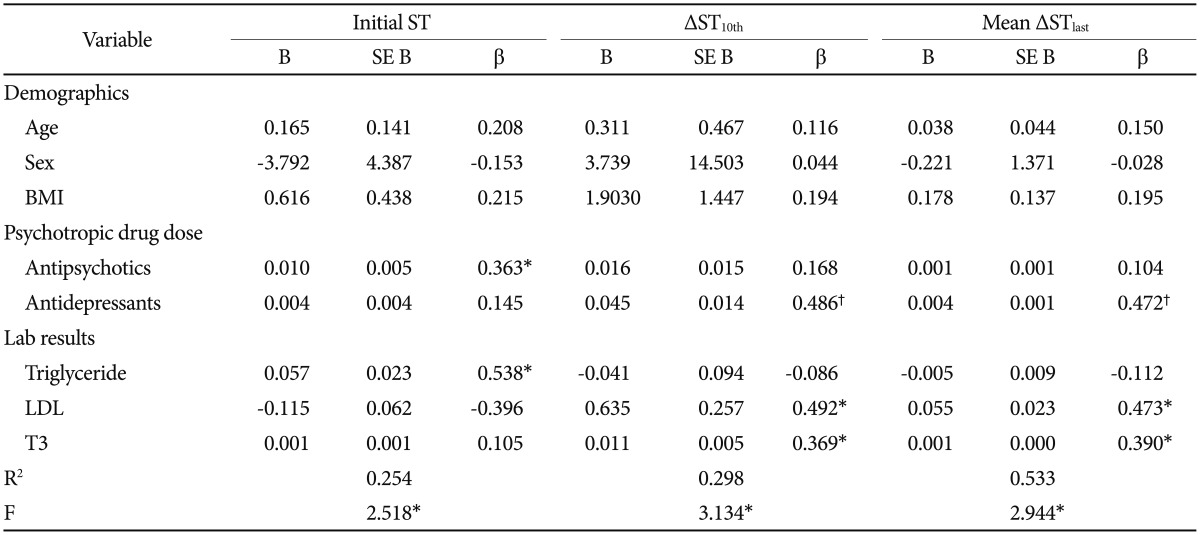
Of the 43 patients, 40 used at least one antipsychotic drug, 12 used more than one antidepressant, and 3 did not take any psychotropic medication while undergoing ECT (Table 2). The total CPZ equivalent dose of antipsychotics (B=0.363, p<0.05) was positively associated with initial ST. The total FXT equivalent dose of antidepressants, on the other hand, was positively associated with ΔST10th (B=0.486, p<0.01) and mean ΔSTlast (B=0.472, p<0.01) (Figure 1).
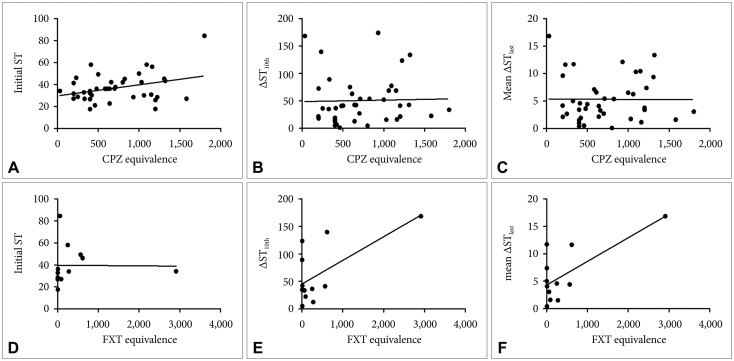
Psychotropic drug doses and their association with ST. A: Antipsychotics and initial ST. B: Antipsychotics and ΔST10th. C: Antipsychotics and mean ΔSTlast. D: Antidepressants and initial ST. E: Antidepressants and ΔST10th. F: Antidepressants and mean ΔSTlast. Variables: ΔST10th, difference in seizure thresholds between first and 10th sessions, mean ΔSTlast: mean difference in seizure thresholds between first and last sessions. ST: seizure threshold in jaules, CPZ: chlorpromazine, FXT: fluoxetine.
Effect of combination therapy on ST
We divided the subjects into four subgroups to further analyze the effects of drug combinations: 11 patients took only one antipsychotic medication, 6 patients took one antipsychotic in combination with antidepressants, 17 patients took multiple antipsychotics, and 6 patients took multiple antipsychotics in combination with antidepressants (Table 2). We performed an ANCOVA for initial ST, ΔST10th, and mean ΔSTlast and adjusted the results for age, sex, and BMI. The initial ST was highest in the group using multiple antipsychotics in combination with antidepressants (45.23±23.02) and lowest in the group using only one antipsychotic (32.89±6.16). ΔST10th and mean ΔSTlast showed similar results (31.14±23.50 vs. 74.22±50.36, and 3.52±2.72 vs. 6.67±4.33, respectively). Those results suggest that use of multiple drugs is associated with higher initial ST and greater increases of ST during ECT (Table 3). However, those differences were not statistically significant.
Laboratory test results and ST
Lab results were significant. Initial ST was positively associated with serum triglyceride levels (B=0.390, p<0.05). ΔST10th showed positive associations with serum low density lipoprotein (LDL) (B=0.412, p<0.05), and serum triiodothyronine levels showed associations with mean ΔSTlast (B=0.326, p<0.05). See Table 4 for a summary of all regression analyses.
DISCUSSION
Optimal electrical stimulus is critical for inducing therapeutic seizures in ECT. As previous studies suggest, energy levels exceeding ST are necessary to achieve proper therapeutic effects.345678 Our research focused on the effects of psychotropic drugs on initial ST and the shift of ST during ECT. We found that both antipsychotics and antidepressants affect the increase of ST, and their associations seem to have dose-dependent patterns. Higher doses of antipsychotics are associated with higher initial ST, and higher doses of antidepressants are associated with larger shifts of ST during the course of treatment.
It was traditionally thought that dopamine agonists could provoke and modulate seizures, increasing the number and severity of attacks.21 One Japanese study investigated the effects of antipsychotic drugs on epilepsy patients and found that the group taking antipsychotic agents tended to have fewer seizure incidents than controls.22 Dopamine antagonists, however, showed inconsistent effects in provoking seizures. Some studies suggested that antipsychotics have dose-dependent proconvulsive characteristics.23 Several articles particularly pointed to the epileptogenic properties of clozapine.242526 On the other hand, consistent with our results, other studies reported possible anticonvulsant effects for antipsychotic medication, especially second-generation drugs.1517 A recent study found antiepileptic effects of aripiprazole during pharmacologically or electrically induced seizures in mice.27
The exact mechanisms by which antipsychotics affect ST remain unknown, but some plausible explanations are possible.
First, dopamine itself is known for its possible seizure-evoking properties. Dopamine, mainly through D1 receptors in the midbrain, has been shown to have proconvulsant effects.28 Animal studies confirmed that injection of D1 agonists can stimulate convulsions, just as cholinergic agonists do.293031 The proconvulsive effects of dopamine agonists have also been shown to increase the severity of electrically induced seizures.32 Antipsychotic drugs might counter such actions through their dopamine antagonistic properties, which might increase ST.
Second, antipsychotics might have anticonvulsant effects via regulation of gamma-aminobutyric acid (GABA) transmission. GABA is already known to play an important role in elevating ST, as shown by medications such as barbiturates or benzodiazepines.3334 ECT itself leads to higher ST and increased GABA levels as sessions progress, adding additional weight to the link between GABA and anticonvulsant effects.35 Antipsychotics also induce an increase in intracerebral GABA concentrations. A study on rats showed that the administration of antipsychotic drugs led to a decrease in the density of GABA receptors, thereby indicating increased activity in the GABAergic system.36 How antipsychotics affect GABA is not yet clear, but animal studies suggest that dopamine antagonists, the main mechanism for most modern antipsychotics, seem to decrease the inhibitory effects of GABA antagonists, thereby leading to increased GABA activity.37 Primate studies have also revealed that GABAergic neurons express D4 receptors in various brain regions, including the cortex, hippocampus, basal ganglia, and substantia nigra, contributing to the idea that dopamine plays a role in GABAergic modulation.38 More recent in vitro studies suggest that dopamine has a biphasic effect on GABA: D2 agonists induce a rapid but short decrease in excitability, whereas D1 agonists lead to a slower but longer increase.39 This finding coincides with previous animal studies that used D1 receptor antagonist on rat brains to observe the consequent decrease of GABA release from striatal terminals.4041 In vitro experiments supported the idea of D1-mediated excitatory influence and D2-mediated inhibitory action on GABA, but they also suggested a third excitatory pathway that does not depend on calcium channels, unlike the two previously mentioned pathways.42 Those results imply that antipsychotics could have antiepileptic effects through D2 antagonistic properties.
Third, the choice of antipsychotic medication might have affected outcomes directly. Previous reviews suggest that chlorpromazine and clozapine have higher risks for inducing seizures, whereas newer drugs such as risperidone, quetiapine, and olanzapine have relatively low risks.4344 A study examining the EEG recordings of psychiatric patients also supports those results, stating that EEG abnormality risks vary among antipsychotic agents from clozapine at 47.1% to quetiapine at 0.0%.45 Most patients in our study took second-generation antipsychotics. Almost all patients had quetiapine or olanzapine prescribed as their primary medication. Amisulpride, clozapine, risperidone, and other drugs were used in much lower numbers. No patient took chlorpromazine during the study. This preference for low-risk drugs might have affected our study in several ways, although the exact reason for the difference in seizure-inducing risk remain to be unveiled. The discrepancy between first- and second-generation medications might result from the different receptors that the drugs target. Modern drugs are mostly serotonin-dopamine antagonists, differentiating from classical agents through their occupancy of 5-HT receptors.46 This action gives second-generation drugs their anti-depressive properties and might also contribute to anticonvulsive effects through GABAergic modulation.
Another major finding of our study is the effect of antidepressants on ST during ECT. We discovered that antidepressants alter the shift of ST during the course of ECT sessions, resulting in larger and more drastic ST rises. Previous reports on the topic were controversial to some extent, showing mixed results. Antidepressants were traditionally thought to have the rare consequence of increased seizure risks.47 Previous reports revealed that risks for developing seizures in patients taking newer antidepressants is as low (0.0–0.4%) as in the general population (0.07–0.09%).48 More recent animal studies, however, emphasized the possible antiepileptic effects of antidepressants: Rats under long-term exposure to antipsychotics and antidepressants were observed for seizure development, and both fluoxetine and duloxetine exhibited anticonvulsant effects.49
Although the exact mechanisms are unknown, there are possible explanations for the effects of antidepressants on ST. GABAergic regulation caused by changes in serotonin levels might be the first plausible reason. SSRIs, among other classes of antidepressants, were reported to upregulate GABA receptors in animal models and clinical studies.5051 Other studies also found that serotonin stimulates GABAergic interneurons directly.5253 5-HT2 and 5-HT3 receptors are responsible for the release of GABA from GABAergic interneurons in the hippocampal region and dentate gyrus, respectively.54 Hence, increased serotonin concentrations lead to higher GABA levels, resulting in stronger anticonvulsant effects. Evidence for such effects is also seen in an animal study performed on epileptic rats and through studies on endogenous serotonin or acute administration of selective serotonin reuptake inhibitors in clinical settings.555657 This hypothesis is also supported by the fact that anticonvulsants such as carbamazepine increase the release of serotonin in patients undergoing pharmacotherapy.17
Second, norepinephrine (NE), a neurotransmitter commonly modulated by antidepressants, also seems to contribute to seizure control. Previous studies indicated that NE target areas, such as the stria terminalis or hippocampus, show increased GABAergic activity when exposed to chronic stress.58 An animal model promoted this idea by showing that the administration of reboxetine, a powerful NE reuptake inhibitor, increases GABAergic transmission in the limbic areas of rats.59 Similar conclusions were obtained in other animal studies: increased GABA concentrations occurred in the hypothalamic and hippocampal areas of rats following stimulation by NE.6061 This GABA increase can result in higher ST, as explained above.
Third, evidence suggests that NE can affect seizures in other ways than by mediating GABA levels. An animal study reported that mice engineered to lack NE showed greater seizure susceptibility than control groups, also displaying higher severity and mortality rates from induced seizures.62 The role of NE in epilepsy was initially depicted through a kindling model, reproduced by engineered rats, in which NE seemed to delay the onset of seizure ‘kindling’ by the amygdala.6364 More recent studies have revealed that NE also counteracts the development of epileptic circuits and even increases neuronal damage caused by epileptic states.65
Apart from the effects of psychotropic drugs, our study also found that higher levels of serum triglycerides correlated with higher initial ST, and higher serum LDL levels correlated with higher ΔST10th values. Related ideas can be traced back to the classical ketogenic diet designed for children with intractable epilepsy. A diet of high fats and low carbohydrates is expected to be ketogenic and increase serum ketone levels. Ketosis is anticipated to activate the tricarboxylic acid cycle and increase GABA synthesis, leading to increased antiepileptic effects.6667 Correlations between high cholesterol levels and raised ST were also observed in an animal study on epileptic monkeys.68 We did not examine serum ketone levels, which is one of the limitations of our study.
Modern drugs offer treatment choices of great efficacy to most clinicians. However, the number of treatment-resistant cases is increasing, making ECT an attractive, if not mandatory, treatment option.69 The American Psychiatric Association Task Force on ECT advises practitioners to stop concomitant use of psychotropic drugs to prevent possible adverse effects,7071 although reviews show that most psychiatric patients ultimately end up receiving more than two psychotropic medications.72 Still, because energy levels beyond ST usually accompany more adverse effects, discontinuation of psychotropic drugs has been a customary habit for many clinicians.73 That tradition is questionable because recent reports suggest that ECT induces 5-HT receptor sensitization, possibly leading to increased drug efficacies in treatment-resistant patients.74 Future treatment-resistant cases might arise, forcing clinicians to reach out for ECT with concomitant poly-pharmacy. Previous research suggests that use of antipsychotic and antidepressant drugs can lower ST and increase seizure risks in those situations. Our results, on the other hand, imply that modern drugs can actually raise ST, unlike most classical drugs. These findings can help predict delicate changes in ST, providing hints for safer treatment techniques with fewer side effects and more effective results.
The strength of this study is that we considered both antipsychotics and antidepressants in our search for possible effects on ST during ECT. Our research also has several limitations. First, the population size was small, and the proportion of patients taking antidepressants was smaller than that of patients taking antipsychotics. Larger populations could lead to more precise results. Second, we used equivalent dose calculations to compensate for the variety of psychotropic medications, but no universal guideline for such conversions has been published. Nevertheless, those conversions are widely used in clinical practice. Third, one of our participants was taking bupropion as an antidepressant although there are studies claiming that bupropion lowers seizure threshold. The small dose (300 mg) is less likely to make a big difference but this might have affected our results. Fourth, previous studies described about the correlations between age and seizure thresholds. Our study also showed positive correlations between age and seizure threshold shifts, but the results were not shown to be significant. We believe that a larger number of participants would have resulted in more significant outcomes.
In conclusions, this study identified factors that affect ST during ECT, specifically focusing on the effect of psychotropic drugs. We found that larger doses of antipsychotics are associated with higher initial ST, whereas antidepressants are associated with stronger shifts of ST, resulting in a steeper increase. The number and amounts of concomitantly used psychotropic drugs should always be kept in mind because titrating for ST is crucial for optimal outcomes. Higher drug doses might demand higher initial energy levels and even larger titration intervals to obtain the best treatment results. Our findings provide a basis for creating safer and more efficient ECT protocols.
Acknowledgments
This study was supported by a grant from the Korea University, Republic of Korea (K1512631).