Serum Cytokine Levels in Major Depressive Disorder and Its Role in Antidepressant Response
Article information
Abstract
Objective
Cytokines have been reported to have key roles in major depressive disorder (MDD). However, much less is known about cytokines in MDD and antidepressant treatment due to the diversity of cytokines and the heterogeneity of depression. We investigated the levels of cytokines in patients with MDD compared with healthy subjects and their associations with antidepressant response.
Methods
We investigated the changes of several cytokines (eotaxin, sCD40L, IL-8, MCP-1alpha, TNF-alpha, INF-gamma and MIP-1alpha) by Luminex assay in 66 patients with MDD and 22 healthy controls. The antidepressant response was assessed by 17-item Hamilton Rating Scale for Depression.
Results
We found the levels of sCD40L (p=0.001), IL-8 (p=0.004) and MCP-1 (p=0.03) of healthy controls were significantly higher than those of depressive patients. However, the level of eotaxin and TNF-alpha were not associated with MDD. In addition, we found the level of MCP-1 was significantly changed after antidepressant treatment (p=0.01).
Conclusion
These findings suggest the roles of cytokines in MDD are complex, and could vary according to the individual characteristics of each patient. Further studies regarding the relationship between cytokines and MDD will be required.
INTRODUCTION
Major depressive disorder (MDD) is the most common of serious psychiatric disorders.1 The connection of MDD and the dysregulation of the immune system has become apparent.23456 It has been found that cytokines may play an important role in this connection.7 Cytokines are a diverse group of small proteins that are regarded as the hormones of the immune system.8 Cytokines affect brain function by supporting neuronal integrity, neurogenesis, and synaptic remodeling.9 Cytokines also have an effect on neurotransmitter systems and the neurocircuit, inducing behavioral alternations.10111213
Numerous studies have reported the increase of proinflammatory cytokines: IL-1, IL-6, tumor necrosis factor (TNF)-alpha, and prostaglandin E2 (PGE2), in major depressive disorder.14151617 A study with a cerebrospinal fluid assay reported that IL-6 concentrations correlate with the severity of depression.18 A recent meta-analysis showed that the concentrations of cytokines (IL-1beta, IL-6, and TNF-alpha) are decreased after antidepressant treatment.19
A series of meta-analyses confirmed the correlation between pro-inflammatory cytokines (TNF-alpha and IL-6) and MDD;71420 however, the role of other cytokines in MDD remains unclear. In addition, ethnicity may play an important role in the relationship between the levels of cytokines and MDD, and relatively few studies have been conducted in the Asian population.7 In this study, we investigated cytokines that have been known to have a role in psychiatric disorder in Korean population with MDD.
Our primary hypothesis is that the levels of cytokines would differ between the patients with MDD and healthy subjects. Secondary, we tested their associations with antidepressant responsiveness and the severity of depression. Additionally, we investigated their changes due to antidepressant medication.
METHODS
Subjects
Sixty-six Korean patients with MDD were recruited from the clinical trials program of the Samsung Medical Center Geropsychiatry and Affective Disorder Clinics (Seoul, Korea). Patients fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), criteria for major depressive episode.2122 Diagnoses were confirmed by a board certified psychiatrist on the basis of an initial clinical interview and a structured research interview.2324 A minimum baseline 17-item Hamilton Rating Scale for Depression (HAM-D) score of 15 was required.25 Exclusion criteria were pregnancy, significant medical conditions, abnormal laboratory baseline values, unstable psychiatric features (e.g, suicide attempt), histories of alcohol or drug dependence, seizure, neurological illnesses, including significant cognitive impairment, or concomitant Axis I psychiatric disorders (schizophrenia, bipolar affective disorder, primary diagnosis of adjustment disorder, or posttraumatic stress disorder). No patient met the DSM-IV criteria for the specifier ‘Severe with Psychotic Features’, and none had received psychotropic medication with the current episode. In addition, no patients had received psychotropic medication within 4 weeks. Twenty-two healthy volunteers without histories of psychiatric illness were recruited by advertisement. A psychiatrist screened the control group with an interview and then biochemical tests were administered. Volunteers with a family history of mood disorders or evidence of inflammatory illness (flu, allergic disease, or dermatitis) or immunopathies were not included. The protocol was approved by the ethics review board of Samsung Medical Center, Seoul, Korea.
Procedures
Patients received an antidepressant monotherapy for 6 weeks. SSRIs [escitalopram (n=31), sertraline (n=2), paroxetine (n=11), fluoxetine (n=1)] and mirtazapine (n=21) were chosen by clinician based on the anticipated adverse effects and the symptomatic characteristics of patients. Dose titration was completed within two weeks to check compliance. The HAM-D scores26 were obtained by a single trained rater every two weeks. A response was defined as a decrease in HAM-D score of 50% or more at week 6.27 The rater and laboratory workers were blinded to the purpose of this study. In addition, cytokine data were not disclosed to the rater and the HAM-D scores were not exposed to the laboratory workers.
Determination of cytokine levels
Through a literature survey based on their likely importance for the psychiatric disorder, we initially selected 19 cytokines (exotaxin, sCD40L, IL-1a, IL-1b, IL-2, IL-4, IL-7, IL-8, IL-10, IL-12, FGF-2, MCP-1, TNF-alpha, alpha2-macroglobulin, Apoprotein AI, Apoprotein E, C3, INF-gamma and MIP-1alpha). We evaluated the analytical performance of the commercially available assay kits, Human Cytokine/Chemokine Magnetic Bead Panel 1 and Human Neurodegenerative Disease Panel 1 (Millipore, Billerica, MA, USA). Seven cytokines (eotaxin, sCD40L, IL-8, MCP-1, TNF-alpha, INF-gamma, and MIP-1alpha) were finally selected in the consideration of precision, calibration range and the limit of quantification of the assay method. These inflammatory markers have been shown to be relevant in psychiatric diseases.714152829 Venous blood was drawn at baseline and at week 6. After serum isolation by centrifugation, the samples were stored at -80℃. Inflammatory markers were measured using 25 µL of serum with a Luminex 200 analyzer (Luminex Corporation, Austin, TX, USA) with the Human Cytokine/Chemokine Magnetic Bead Panel 1.
Markers for which more than 10% of the samples had values below limit of quantitation (LOQ) at baseline were not included in the analysis.30 Two cytokines were excluded from the analysis because they frequently had values below the LOQ (53.4% for INF-gamma, and 67.0% for MIP-1alpha). Eotaxin (none), sCD40L (none), IL-8 (1.1%), MCP-1 (none), TNF-alpha (8.0%) were included in the analysis. For each marker retained for analysis, samples with undetectable values were given the value zero.
Statistical analysis
Continuous variables are presented as median and interquartile range. The Wilcoxon rank-sum test was employed to compare these variables between healthy control subjects and patients or the responsive and non-responsive groups. Categorical variables were presented as frequencies and proportions, and tested with Fisher's exact test. Median regression was employed to compare non-normally distributed continuous variables adjusting for other variables. The Wilcoxon signed-rank test was used to compare the cytokine values of pre- and post-antidepressant treatment. Relationships between two continuous variables were tested by Spearman's correlation analysis. p-values were corrected by Bonferroni's correction for the multiple association tests of cytokines and noted as corrected p. All differences were considered to be significant at p<0.05. Statistical analysis was undertaken using STATA SE 10.0 for Windows (College Station, TX, USA).
RESULTS
Clinical and demographic characteristics
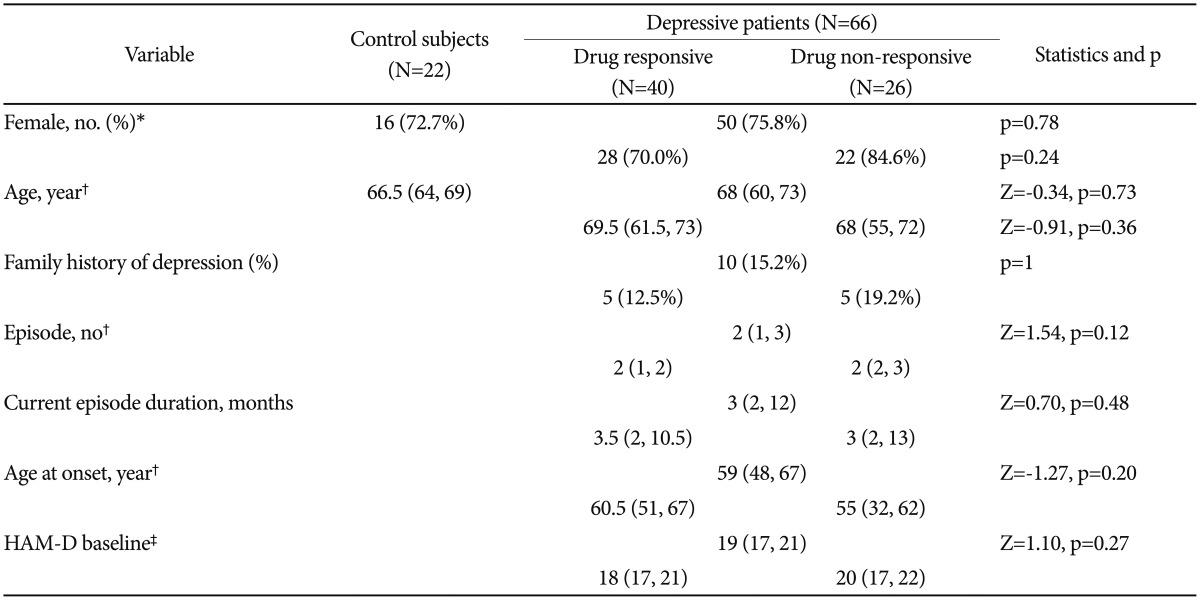
Clinical and demographic characteristics are shown in Table 1. There were no notable differences between the control subjects and patients with respect to age and sex. The rate of response to antidepressants was 40 of 66 patients (60.6%). A family history of depression was present in 15.2% of the patients. The median baseline HAM-D score was 19 (interquartile range: 17–21), indicating moderate to severe depression. Age, sex, episode number, current episode duration, age at onset, and baseline HAM-D score were not associated with antidepressant responsiveness. In addition, choice of drug had no effect on the rate of response (p>0.05). The median HAM-D score gradually decreased from 19 to 9 (week 2: 15,12131415161718 week 4: 12,91011121314151617 week 6: 967891011121314).
Serum cytokine levels and diagnosis
Baseline plasma concentrations of cytokines in the participants are shown in Table 2 and Figure 1. The median value of sCD40L of healthy controls was higher than that of depressive patients (44950 pg/mL, 2880–66700 pg/mL vs 19700, 5990–40800 pg/mL, respectively; corrected p=0.01). This difference was maintained after adjusted for age and sex [adjusted p=0.001, 95% confidence interval (CI)=14036.24–43266.99]. IL-8 (corrected p=0.01) and MCP-1 (corrected p=0.002) were significantly associated with a diagnosis of major depressive disorder. The levels of IL-8 and MCP-1 were higher in healthy controls when compared to depressive patients. These associations were robust after adjusted for age and sex (IL-8: adjusted p=0.004, 95% CI=3.09–10.73, MCP-1: adjusted p=0.03, 95% CI=44.71–254.38). The levels of these cytokines did not differ with sex, old age (>60), late onset (>60) and recurrence (number of episode ≥2).

Baseline serum concentrations of cytokines in major depressive patients (N=66) and healthy controls (N=22)

Box plot: baseline plasma concentrations of cytokine in major depressive patients and healthy controls. A: Eotaxin, B: sCD40L, C: IL-8, D: MCP-1, and E: TNF-alpha. p-values were calculated by median regression analysis after adjusted for age and sex; Bonferroni correction was used for the five cytokines. sCD40L: soluble CD40-ligand, IL-8: interleukin 8, MCP-1: monocyte chemoattractant protein-1, TNF-alpha: tumor necrosis factor alpha.
Serum cytokine levels and antidepressant treatment
We found no association between baseline cytokine concentration and antidepressant response (Table 2). In addition, changes of cytokine concentrations after 6 weeks of antidepressant treatment were not associated with responsiveness.
We found the level of MCP-1 significantly increased after antidepressant treatment (corrected p=0.01) (Table 3). This association was observed in both the response (corrected p=0.04) and non-response (corrected p=0.04) groups. Moreover, the significance of difference in MCP-1 concentration between the healthy controls and depressive patients disappeared after antidepressant treatment (p=0.06, 95% CI=-2.34–168.80).
Serum cytokine levels and severity of depression
There was no significant correlation between HAM-D score and cytokine concentration at baseline and at week 6. The HAM-D score changes did not correlate with cytokine level changes after antidepressant treatment.
DISCUSSION
In this study, we investigated the association between depression response to antidepressant medication, and several inflammatory cytokines. We identified the association between diagnosis of depression and three cytokines (sCD40L, IL-8 and MCP-1), and we found that the level of MCP-1 was significantly increased after antidepressant treatment.
CD40 ligand (CD40L) is a transmembrane glycoprotein structurally related to TNF-alpha.31 CD40 ligand exists in two forms, membrane-bound and soluble (sCD40L). The protein binds to the CD40 receptor and induces the production of cytokines, connective tissue degrading enzymes, and upregulates the inflammatory response.32 This interaction between CD40 and CD40L is known to be an important step in the priming of helper T-cells.33 Leo et al.28 reported that sCD40L levels increased in patients with MDD compared with controls and also found a correlation between the level of sCD40L and the severity of depression as assessed by the HAM-D (Table 4). In addition, they reported that mood improvement with SSRI treatment was associated with a reduction in sCD40L.28 Another study reported by Neubauer et al.34 showed a concordant result, that CD40L was elevated in patients with depression as compared to controls. However, our results showed a diametrical association; the level of sCD40L in healthy controls was higher than that of depressive patients.

Summary of previous studies that investigated the association between depression and the significant cytokines in our study
We can speculate several reasons for the discrepancy. The first is the difference of the illness duration. Previous studies were conducted during the first episode of depression; however, in our study, depressive patients (72.7%) were experiencing their second or a later episode of MDD. It has been suggested that cytokine-induced neurochemical alterations might vary with the number of depressive episodes.35 Griffiths et al.36 suggested that the recurrence of depression might involve chronic cytokine activation. Further studies for CD40L in recurrent depression are needed. Another possible factor for such discrepancy is the proportion of old age patients. Previous studies that reported an elevated level of CD40L in depressive patients included mostly middle-aged patients.2834 Our patients were mostly elderly (age >60 years, 74.2%), and a large portion of them had late-onset illness (age of first onset >60 years, 45.5%). Several studies have suggested that the age at onset can vary in different subtypes of depression, especially in terms of heritability.3738 In addition, previous studies reported that proinflammatory cytokines increase in healthy elderly people,394041 although, we could not find a significant relationship between age, age on onset and sCD40L in our study subjects. Another consideration is that our study was conducted in Asian population. Further studies with clinically homogeneous samples in recurrent depression and in an elderly group or various ethnic groups will be required.
Previous studies that investigated the association between IL-8 and depression reported inconsistent results (Table 4). Simon et al. found elevated level of IL-8 in depression,42 but three other studies found no significant association.194344 In contrast, Lehto et al. reported a decreased level of IL-8 in depression, which was in agreement with our results.45 IL-8 has been known as a neutrophil chemotatic factor;46 however, the detailed functions of this cytokine are complex. High circulating levels of IL-8 act to decreased the infiltration of neutrophils to the inflammatory site, performing pro- or anti- inflammatory roles, depending on the concentration.8 Therefore, there was a possibility that the association between IL-8 level and depression could be changed according to the study population.
Relatively few studies have investigated the association between MCP-1 and depression. Lehto et al.45 and Grassi-Oliveria et al.47 found that depression was associated with lower MCP-1 concentrations compare to healthy controls, but others reported contrary results (Table 4).4248 Our results, finding lower levels of IL-8 and MCP-1, suggest a reduced level of chemokines in depression. The detailed mechanism for this association is unclear. The neuroprotective function of neuronal chemokines,4950 and their dopaminergic activity-enhancing effect in the central nervous system could be possible explanations. However, limitations to our interpretation should be noted because our results were obtained from peripheral blood.
Sutcigil et al.48 reported that the level of MCP-1 decreased after sertraline treatment. However, we found an increase in MCP-1 levels following antidepressant treatment. The major difference between our result and the previous study is the number of episodes that patients experienced. Sutcigil et al. included only first episode patients in their study, but, as noted above, most of our study patients were recurrent depression. Although, we could not find a significant relationship between episode number and the level of MCP-1 in our study subjects, the difference in the antidepressants used may have also contributed to this discordance. Interestingly, the directions of change in both studies were toward those of the healthy controls. The study of Sutcigil et al. showed a higher level of MCP-1 in depressive patients compare to healthy controls, and the difference decreased after treatment. We found a decreased level of MCP-1 in depressive patients, and it was normalized toward the level of healthy controls after antidepressant treatment. The antidepressant effect of COX-2 inhibitors that block production of prostaglandin E and proinflammatory cytokines were investigated in several clinical trials.515253 In one study, combination therapy of COX-2 inhibitor (celecoxib) and sertraline reduced level of serum IL-6 levels in patients with MDD, and reduction of the severity of depressive symptoms and reduction of the level of serum IL-6 were significantly correlated.53 Further studies that investigate the effect of the combination treatment with COX-2 inhibitor on the level of MCP-1 could be helpful.
We found no association between the level of eotaxin or TNF-alpha and depression. These negative results were inconsistent with recent studies for eotaxin4754 and a meta-analysis of TNF-alpha.7 However, our findings are in line with other reports conducted in elderly patients.555657 Additional studies adjusted for age with larger sample sizes are required.
Several limitations were present in our study. Our sample was relatively small. The cross-sectional design for association between depression and cytokines precludes conclusions about causality. We could not consider potential variables, such as smoking, lifetime use of anti-inflammatory or analgesic drugs, minor medical illness, and body mass index. Another consideration is that because our patients were mostly elderly due to the clinical setting of patient enrollment, the generalizability of our results to other age groups may be limited.
Overall, we found the associations between sCD40L, IL-8, MCP-1, and depression. In addition, we showed that the level of MCP-1 was significantly increased after antidepressant treatment. Our results imply that the characteristics of study population are important, and the roles of cytokines in depression are sophisticated and nuanced. The relationship between cytokines and depression remains to be clarified. Further studies concerning covariance with age and recurrence are required.
Acknowledgments
This work was supported by a grant of the Korean Health Technology R&D Project from the Ministry of Health & Welfare, Republic of Korea (A110339, HI14C2071). These funding sources were not involved in the creation of the study protocol, data analysis, or in writing the manuscript.