Treatment-Resistant Depression Entering Remission Following a Seizure during the Course of Repetitive Transcranial Magnetic Stimulation
Article information
Abstract
Major depressive disorder is often resistant to antidepressant treatment. Repetitive transcranial magnetic stimulation (rTMS) has been used in treatment-resistant depression (TRD). Known adverse events of rTMS include transient headache, local pain, syncope, seizure induction, and hypomania induction. This report outlines a patient with TRD who unexpectedly improved following a seizure during the course of rTMS, which has never been reported.
INTRODUCTION
Depression is a common mental disorder with an annual prevalence of 6.6–16.2%; the lifetime prevalence is approximately 40%.1 Depression is associated with high rates of morbidity and mortality, and psychosocial impairment.23 When assessing depression treatment outcomes, remission is typically defined in a practical sense as low or absent symptoms,4 usually based on assessment scales such as the Hamilton Rating Scale for Depression (HAM-D)5 or Montgomery-Asberg Depression Rating Scale (MADRS).6 Treatment-resistant depression (TRD) is defined as a lack of remission after at least two adequate trials of treatment with different classes of antidepressants.78 TRD is relatively common in clinical practice, with 50–60% of patients not achieving remission following 8–12 weeks of antidepressant treatment.9
To manage TRD, several pharmacotherapeutic approaches can be used, including the addition of another agent or a switch to another antidepressant.1011 Several recent studies have confirmed the efficacy of transcranial magnetic stimulation (TMS) in TRD.1213 TMS uses nerve depolarization principles such as a general electrical stimulation method in which the magnetic field formed is transformed into an electric field of the appropriate intensity and time.14 Repetitive TMS (rTMS) can change the excitement level of the cerebral cortex in a short time period; the changing excitement level is affected by several factors, such as the number, level, frequency, and total number of repeated magnetic stimulations. Of these, the frequency of stimulation is crucial.15 Low frequency (<1 Hz) suppresses the excitement level of the cerebral cortex, while high frequency (>5 Hz) increases it.16
The safety of TMS has been reported in recent meta-analyses.1718 Known complications include hypomania induction, syncope, transient headache, local pain, hearing changes, and burns from the electrodes. Seizure induction has also been reported, with an estimated prevalence of seizures induced by high-frequency rTMS of less than 1%.19 Because electroconvulsive therapy is effective at treating depression2021 and electroconvulsive seizures themselves might counteract depression,2223 a seizure induced by rTMS may have beneficial effects in the treatment of depression. However, there is no report on improvement in a case of depression following a seizure event after rTMS. Here, we present a patient with TRD who improved following a seizure during rTMS treatment.
CASE
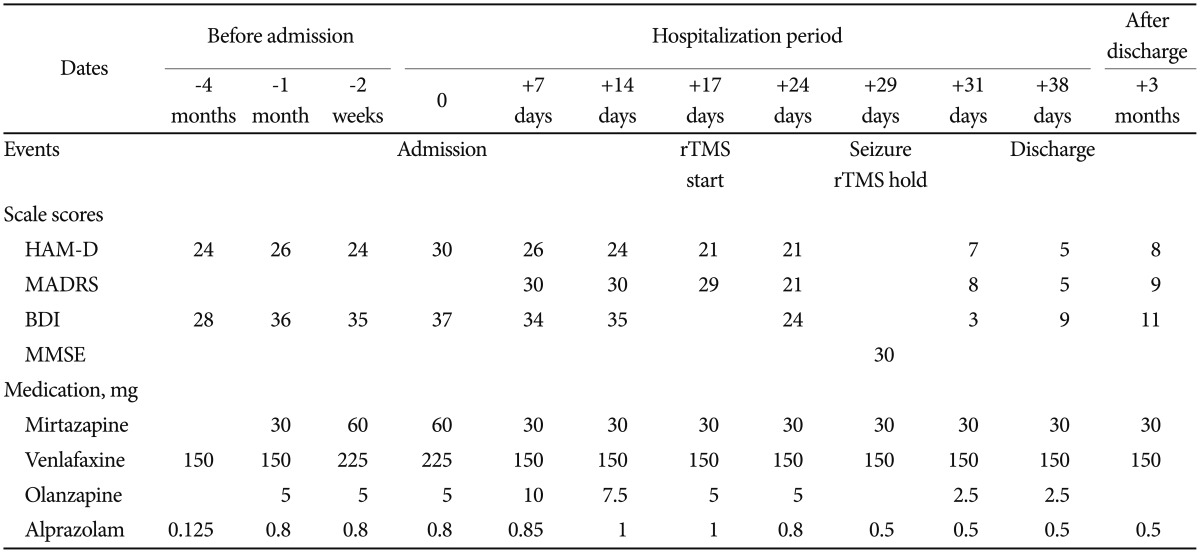
A 51-year-old woman had suffered from major depressive disorder that had waxed and waned for the last 13 years, with five depressive episodes and three hospitalizations. She had no previous manic or hypomanic episode. She had no personal or family histories of seizure. Her assessment scale scores and medications used for treatment are summarized in Table 1. Four months before her latest hospitalization, her depressive symptoms were exacerbated, and she did not respond adequately despite outpatient treatment that included full doses of two different classes of antidepressants (mirtazapine and venlafaxine) for 2 months, augmented with an atypical antipsychotic (olanzapine). She attempted suicide and was hospitalized. At the time of hospitalization, her scores on the Beck Depression Inventory (BDI)24 and HAM-D were 37 and 30, respectively, suggesting severe depression pathologies.
In the hospital, her drugs were adjusted and cognitive behavior therapy was given. However, there was less than 25% improvement based on the depression assessment scales after 2 weeks of treatment. Since the patient had taken full courses of antidepressants of more than two types and different classes over 10 weeks, we diagnosed her condition as TRD according to various TRD guidelines, including the Committee for Proprietary Medicinal Products guidelines and Massachusetts General Hospital Staging Method.910 We decided to administer rTMS based on the TRD treatment guidelines.25
The treatment protocol was as follows: at 120% of the motor threshold and a pulse frequency of 10 pulses per second, we administered 80 stimulation cycles of 4 seconds on (active stimulation) and 26 seconds off (no stimulation), resulting in 3,200 pulses per treatment session. After the 11th rTMS, she showed slight improvement, albeit not meaningful clinically. The next day, just before the 12th TMS session, her left lip suddenly quivered for 30 seconds and she drooled. The medical team asked her to spit out a candy she was eating. However, she did not follow these directions, and drooled with a blank facial expression. Subsequently, she could not recall this event. We assessed her condition as an absence seizure, a serious adverse event of rTMS, and decided to stop rTMS treatment. We performed sleep EGG immediately and found dysmorphic theta to delta range slow waves in both cerebral hemispheres with frontal dominancy. Unexpectedly, the next day, the patient's psychopathology changed dramatically. Her mood and cognitive symptoms seemed to improve abruptly and she said that she felt much better and could enjoy watching television. Based on the BDI, HAM-D, and MADRS, her depression had improved to remission status. We believe that her depression improved very rapidly after the seizure. The patient showed no other adverse events related to the seizure, such as cognitive dysfunction, nausea, vomiting, or headache. We repeated her sleep EGG one week later and found theta to delta range slow waves in both cerebral hemispheres with frontal dominance, but no abnormal epileptiform discharges. She was discharged in stable condition on the 38th day of admission. Three months after discharge, she remains in stable condition with no further seizures on a stable medication dose.
DISCUSSION
A TMS-induced seizure is a rare, but significant, adverse event in patients with no history of seizures, even when rTMS is used within the suggested guidelines. Therefore, TMS needs to be supervised by a clinician in a facility capable of responding to a potential seizure quickly.2627 In this report, we present a case of TRD that unexpectedly improved following a seizure during the course of rTMS. As far as we aware, this has never been reported.
There are several possible explanations for the patient's rapid improvement. First, the psychotropic medications may have had a cumulative effect. The patient received full doses of two different antidepressants for 10 weeks after her exacerbation in this episode, but there was minimal symptom change during this period. Therefore, it is difficult to believe that her sudden improvement was due to the effects of these medications. Second, the cumulative effects of rTMS could also be considered. Perhaps the 11 rTMS sessions performed led to her improvement. However, there had been no change in the scores on the depression rating scales up to the 11th rTMS session. Third, the seizure, which was a side effect of rTMS, could have had an effect similar to electroconvulsive therapy (ECT). ECT or magnetic seizure therapy (MST) improves depression by triggering intentional seizures based on convulsive stimulation.202128 ECT affects depression by changing neurotransmitter receptors and second-messenger systems. ECT sessions downregulate postsynaptic β-adrenergic receptors and alter the muscarinic, cholinergic, and dopaminergic neuron systems.29 Remission usually takes 6–12 ECT sessions, but remission of depression has followed ultrabrief pulse ECT.30 Several studies have reported the molecular effects of electroconvulsive seizures (ECS); ECS increases the expression of genes in the brain-derived neurotrophic factor/neural receptor protein-tyrosine kinase/mitogen-activated protein kinase pathway, arachidonic acid pathway, and genes for vascular endothelial growth factor, thyrotropin-releasing hormone, neuropeptide Y, and regulators of neuronal sprouting and neurogenesis.223132 Single and repeat ECS can cause expression of these genes.23 However, the seizures in ECS are generalized tonic-clonic seizures and not the absence seizure that occurred in our case.
This case was reported in order to share our unusual observations of depression improvement after a seizure that occurred as a side effect of rTMS treatment. Psychotropic drugs, especially antidepressants and antipsychotics, may reduce the seizure threshold and provoke seizures.33 Among psychotropics, bupropion immediate-release (IR) and clozapine have relatively high seizurogenic potential.34 We postulate that seizures caused by the use of psychotropics during rTMS treatment might be used to treat resistant depression. However, this may also lead to the development of an epileptic disorder, as in ECT. This possibility needs more empirical research and verification.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0003).