Association of Alexithymia With Positive Symptoms in Chinese Chronic Schizophrenia Patients With and Without Obesity
Article information
Abstract
Objective
A growing body of research suggests the presence of alexithymia (a form of social cognitive impairment) in patients with schizophrenia (SCZ), which may be related to their psychopathological symptoms. Patients with SCZ exhibit high rates of obesity. Interestingly, studies of the general population have found that alexithymia acts a pivotal role in the development and maintenance of obesity. However, little is known regarding the relationship between obesity, alexithymia, and clinical symptoms in SCZ patients. The study was aim to explore the relationship between obesity, alexithymia, and clinical symptoms in SCZ patients.
Methods
Demographic and clinical data were collected from 507 patients with chronic SCZ. Their symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS), and alexithymia was assessed with the Toronto Alexithymia Scale (TAS).
Results
Compare with nonobese SCZ patients, obese SCZ patients scored higher on PANSS positive symptoms, TAS total score, difficulty identifying feelings, and difficulty describing feelings (all p<0.05). Correlation analysis revealed a significant association between difficulty identifying feelings and positive symptoms in SCZ patients. Further correlation analysis showed that this association was only present in obese SCZ patients (p<0.05).
Conclusion
Obesity may moderate the association between alexithymia and positive symptoms in chronic SCZ patients.
INTRODUCTION
Social cognitive impairment has been present in patients with schizophrenia (SCZ) [1,2], and it has been associated with altered immune system and negative clinical outcomes such as suicidal behavior. Importantly, suicidal behavior may be associated with immune-inflammatory abnormalities [3], and although their causal relationship has not been elucidated, social cognitive impairment has the potential to contribute to the condition and ultimately affect the social and functional outcomes of patients [4]. Therefore, in recent years, the treatment of social cognitive impairment is often used as one of the targets for functional improvement in SCZ patients [5].
Alexithymia, as a type of social cognitive functioning, refers to a lack of emotional self-awareness relative to deficits in the perception of others’ emotions, and usually includes both cognitive and emotional dimensions [6,7]. Alexithymia may be a vulnerable factor in the development of SCZ [8,9], and its features overlap with negative symptoms of SCZ [7,10-12]. Previous studies have attempted to examine the unique patterns of alexithymia in SCZ and their relationship to specific psychopathological symptoms. For example, Gawęda and Krężołek [13] found a significant association between alexithymia and the severity of hallucinations or cognitive deviations in paranoid SCZ patients. However, Fogley et al. [14] alexithymia appears to be an independent structure, unrelated to any psychopathological symptoms. The results are highly heterogeneous and may be influenced by subtypes of SCZ [15]. For example, İnanç et al. [11] found that alexithymia was not associated with negative symptoms, but was significantly negatively associated with positive symptoms only in SCZ patients with deficit syndrome. In addition, alexithymia is often associated with major depression and suicidal behaviors in SCZ patients [15]. Demirkol et al. [10] demonstrated that psychological pain (both direct and indirect) and alexithymia (indirect) may be associated with the risk of suicide in SCZ patients. Unfortunately, it is not associated with a successful therapeutic response, and there is evidence that at low doses, psychopharmacological agents such as buprenorphine are efficacious, well-tolerated, and safe options in reducing depressive symptoms, serious suicidal ideation and non-suicidal self-injury, even in patients with treatment resistant depression [16]. Hence, it is necessary to investigate the relationship between alexithymia and psychopathological symptoms in specific subtypes of SCZ patients.
Patients with SCZ exhibit a high rates of obesity [17], and weight gain is often accompanied by a worsening of positive symptoms [18] and an improvement of negative symptoms [19]. Interestingly, studies on the general population have found that alexithymia plays an important role in the development and maintenance of obesity. Obese individuals have higher levels of alexithymia than normal weight individuals, and have difficulties in identifying feelings and externally oriented thinking [20]. However, there are no published studies showing whether there is also an association between obesity and alexithymia in SCZ patients. Furthermore, it is worth exploring the association of alexithymia with symptoms between obese and nonobese individuals in order to better understand its heterogeneous characteristics and to provide targeted interventions in clinical practice for different subtypes of SCZ patients. Therefore, the basic goals of this study were to explore the relationship between alexithymia and psychiatric symptoms in Chinese SCZ patients and the role of obesity in this relationship.
METHODS
Subjects
We recruited a total of 507 participants (325 males and 182 females) from three psychiatric hospitals in Wuhan, namely the Wuhan Mental Health Center, the Wuhan Xinzhou District Mental Health Center, and the Wuhan Youfang Hospital. Two professional psychiatrists conducted the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) with all participants and confirmed that all of them met the DSM-IV diagnosis of SCZ. All SCZ patients were chronic with a minimum duration of 1 year and a mean duration of 17.72±10.78 years. They were all Han Chinese and ranged in age from 17 to 70 years. All patients were in stable conditions and their treatment regimes were not change for at least the three previous months. With the exception of 8 patients who had no record of taking antipsychotic medication, all patients had been on stable doses of antipsychotic medications for more than six months, with a mean antipsychotic dose (in terms of chlorpromazine equivalents) of 324.34±730.26 mg/day. Among them, 9 subjects were treated with typical antipsychotics, 450 subjects were treated with atypical antipsychotics, and 40 subjects were treated not only with antipsychotics but also with non-antipsychotics. None of the subjects had serious physical illness, serious mental retardation or neurological disorders, and drug and alcohol abuse or dependence. In addition, no female subjects were pregnant or lactating.
Since admission, all patients received three balanced meals per day from the hospital. During their hospitalization, they occasionally supplemented with fruit or snacks from family or friends. In addition, they had the opportunity to do about 1–2 hours of light exercise each day. They walked or ran in the hospital courtyard or went to the gym in their own ward to exercise.
Finally, a thorough physical and laboratory examination was performed on each subject. The Institutional Review Board of the Institute of Psychology, Chinese Academy of Sciences, approved the protocol of this program (H18031). Written informed consent was collected from all subjects.
Psychopathological assessment
Each patient’s psychopathology was evaluated by six experienced psychiatrists using the Positive and Negative Syndrome Scale (PANSS). They were trained in the use of PANSS prior to the start of this study, and their inter-evaluator correlation coefficient exceeded 0.8 in repeated assessments. The PANSS is a commonly adopted scale for assessing the severity of psychopathological symptoms in SCZ patients and consists of three factors: general psychopathology, negative symptoms, and positive symptoms [21]. More recently, a 5-factor model of the PANSS has been developed, which is considered to provide a better understanding of the structure of the PANSS, with a newly extracted depression factor and a cognitive factor. Specifically, the depression factor was calculated from items G2 (“anxiety”), G3 (“guilt feelings”), and G6 (“depression”), and the cognitive factor was calculated from items P2 (“conceptual confusion”), N5 (“difficulty in abstraction withdrawal”), and G11(“poor attention”) [22,23].
Anthropometric measurements
Body mass index (BMI) is a parameter closely related to total body fat and mainly reflects systemic obesity, expressed as the ratio of an individual’s weight to height (kg/m2). Subjects were asked to stand barefoot and their height was measured in meters. An electronic balance was used to evaluate the weight of the indoor light enclosure. According to the Working Group on Obesity in China, obesity was defined as a BMI greater than or equal to 28, while nonobesity was defined as less than 28.
Alexithymia
In this study, the Toronto Alexithymia Scale (TAS)-20 was adopted to evaluate patients’ alexithymia [24]. There were 20 items, ranging from 1 to 5 points (strongly disagree=1 point, disagree=2 points, neutral=3 points, agree=4 points, and strongly agree=5 points), with among which items 4, 5, 10, 18, and 19 being were scored in reverse scored. In addition to the total score, the TAS-20 includes three factors: difficulty in identifying feelings, difficulty in describing feelings, and externally oriented thinking. Concretely, factor 1 includes items 1, 3, 6, 7, 9, 13, and 14; factor 2 includes items 2, 4, 11, 12, and 17; and factor3 includes items 5, 8, 10, 15, 16, 18, 19, and 20. Good validity and test-retest reliability have been reported for the Chinese version of the TAS-20 was reported to have good validity and test-retest reliability, indicating that the scale has good cross-time stability and cross-cultural validation of the scale [25].
Data analysis
In this study, one-way analysis of variance (ANOVA) and χ2 test were performed to compare the differences between the two groups on categorical and continuous variables. First, we used ANOVA to compare the TAS total and its three subscale scores in nonobese and obese patients. Analysis of covariance was then used to compare the TAS scores between the two subgroups by adding significant demographic and clinical variables as covariates. Multiple testing was adjusted by Bonferroni correction. In addition, the association between alexithymia and positive symptoms was investigated by multiple linear regression analysis under different obesity conditions.
All data were statistically analyzed by software IBM SPSS 26.0 (IBM Corp., Armonk, NY, USA) with a significance level of 2-tailed p<0.05. Data in this study are expressed as mean±standard deviation.
RESULTS
Characteristics of SCZ patients with or without obesity
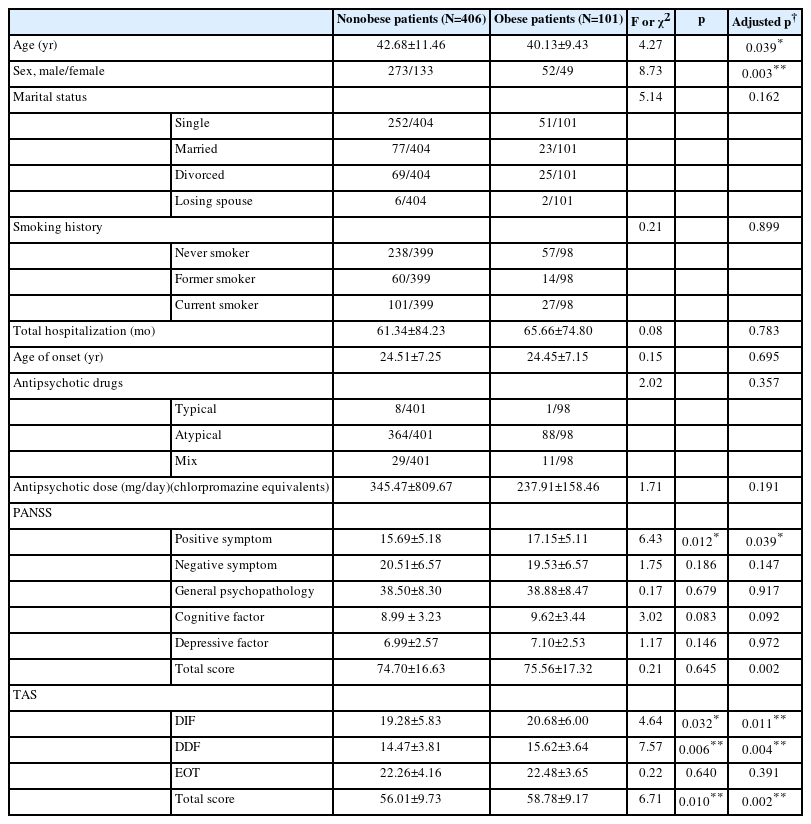
Among all SCZ patients, obesity was significantly higher in females than in males (26.9% vs. 16%; χ2=8.73, p=0.003), indicating that females were more inclined to be obese than males. The age difference between the obese and nonobese patients was also significant, showing that obese patients were younger than nonobese patients (40.13±9.43 vs. 42.68±11.46, F(1,505)=4.27, p=0.039) (Table 1).
Table 1 summarizes the clinical symptoms in both groups. We found that only positive symptoms differed significantly between the two groups, showing that obese SCZ patients had significantly more positive symptoms than nonobese patients (17.15±5.11 vs. 15.69±5.18, F(1,505)=6.43, p=0.012). After adjusting for age and gender as covariates, further ANOVA revealed that a significant difference in positive symptoms was still observed between the two subgroups (F(1,505)=4.28, p=0.039).
In addition, obese patients had higher total TAS score (58.78±9.17 vs. 56.01±9.73, F(1,505)=6.71, p=0.010), difficulty identifying feelings subscale score (20.68±6.00 vs. 19.28±5.83, F(1,505)=4.64, p=0.032), and difficulty describing feelings subscale score (15.62±3.64 vs. 14.47±3.81, F(1,505)=7.57, p=0.006), with a power of 0.58 to 0.77. Nevertheless, the significance of difficulty describing feelings subscale score did not pass Bonferroni correction. After adjusting for age and gender as covariates, there were still significant differences in TAS total score (F(1,502)=9.31, p=0.002), difficulty identifying feelings subscale score (F(1,502)=6.47, p=0.011), and difficulty describing feelings subscale score (F(1,502)=8.20, p=0.004) between the two subgroups. And all of them passed Bonferroni correction.
Relationship between alexithymia and positive symptoms in all SCZ patients
After controlling for age and gender, partial correlations were further used to explore the association between alexithymia and positive symptoms in obese and nonobese SCZ patients (Table 2). Only positive symptoms were significantly positively correlated to difficulty identifying feelings (r=0.124, p=0.005, Bonferroni corrected p<0.05), and borderline significantly correlated with TAS total score (r=0.087, p=0.05, Bonferroni corrected p>0.05). Further multiple regression showed that only difficulty identifying feelings was a significant predictor of positive symptoms (beta=0.128, t=2.606, p=0.009).
Relationship between alexithymia and positive symptoms in obese and nonobese patients
We examined the relationship between alexithymia and positive symptoms in obese and nonobese patients, respectively (Table 3). Only in obese patients, difficulty identifying feelings was significantly positively correlated to positive symptoms (r=0.295, p<0.001), which also passed Bonferroni correction. However, there was no significant association in difficulty identifying feelings and positive symptoms in nonobese patients (r=0.048, p=0.33). Further multiple regression showed that difficulty identifying feelings was independently relate to the severity of positive symptoms in obese patients (beta=0.311, t=3.073, p=0.003) but not in nonobese patients (beta=0.071, t=1.26 p=0.208). These fundings indicate that obesity may be a moderator of the relationship between positive symptoms and difficulty identifying feelings. In addition, glycolipid metabolic parameters were not associated with any symptoms (all p>0.05).
DISCUSSION
It is worth mentioning that obese individuals had significantly more positive symptoms were significantly more serve in obese than in nonobese SCZ individuals, which is was inconsistent with the majority of most previous studies reporting no significant relationship association of between obesity and with positive symptoms [19,26]. Only one published paper found that obese patients had fewer positive symptoms than nonobese patients [27]. In addition, many studies have found that weight gain in patients treated with antipsychotic medication is often tends to be accompanied by improvements in negative symptoms and general psychopathological symptoms [19,26]. However, this study did not support such a conclusion. These differences may be due to a variety of multiple reasons, such as different genetic backgrounds, different stages of illness, the disease, or exposure to different antipsychotic medications. Therefore, the relationship between obesity and clinical symptoms deserves further investigation in longitudinal studies.
Interestingly, we found that the degree of alexithymia was higher in obese individuals than in nonobese individuals. Further analysis of the three factors of alexithymia showed that their manifestations differed between obese and nonobese patients. Compared to nonobese patients, obese patients had higher difficulty identifying feelings and difficulty describing them, but there were no differences in externally oriented thinking, suggesting that obese patients were unable to accurately identify their subjective feelings or describe them to others. These findings were not surprising; difficulty identifying feelings and difficulty describing feelings were simultaneously higher because the verbal expression of emotions depends on the ability to identify them [24]. The emotion-driven eating model suggests that emotional and cognitive processing dysfunctions are responsible for binge eating, which may explain inappropriate eating behaviors [28]. Individuals with mood disorders tend to use eating behaviors to avoid or suppress negative emotions [29]. Thus, as a key component of mood disorders, alexithymia may trigger inappropriate eating behaviors in patients with SCZ, which in turn may lead to obesity.
In contrast to the most previous results showing that there was no relationship between alexithymia and positive symptoms [7,14], the present study found that difficulty identifying feelings in alexithymia was markedly positively related to positive symptoms in chronic SCZ patients, which was only consistent with Maggini and Raballo [30]. In contrast, Gawęda and Krężołek [13] found that hallucinations, but not delusions or general dimensions, were associated with alexithymia. This different result is understandable because cognitive models of psychotic symptoms suggest that hallucinations and delusions may have their own specific cognitive and affective pathways [31]. Alexithymia is one of emotional disorders and its association with positive symptoms may provide a new perspective for improving our positive symptoms.
The most striking result was that positive symptoms were associated with difficulty in identifying feelings only in obese patients, but not in nonobese patients, suggesting that obesity may be a moderator of this relationship. However, it is not clear why this relationship exhibits differently under different obesity conditions. Previous studies have found that obesity is associated with white matter (WM) integrity in SCZ patients, showing that obese patients have lower fractional anisotropy levels in the longitudinal fasciculus, corpus callosum, and corona radiata [32]. It has been suggested that obesity may lead to WM disruption and decrease in structural brain connectivity. Changes in WM have been correlated with psychopathological symptoms [33]. Also, decreased WM integrity in the corpus callosum has been reported to be associated with alexithymia in SCZ patients [34]. Therefore, whether the contribution of obesity to the relationship between alexithymia and psychopathological symptoms is caused by changes in WM may become the focus of future research on potential mechanisms.
Some limitations of this study should be considered. First, our study adopted a cross-sectional design, which that naturally does not allow precludes causal inferences to be made about the relationship between obesity and alexithymia in SCZ patients. Therefore, the results of our study should be considered exploratory. Second, due to the lack of healthy controls, this study could not validate the conclusions regarding the presence or absence of alexithymia in patients with chronic SCZ, nor could it compare the degree of alexithymia in obese and nonobese patients. Third, although the TAS-20 is often used to assess evaluate alexithymia in SCZ patients, as a self-report measure, it depends to some extent on a person’s own mental status, and this ability may be impaired in SCZ patients. Therefore, alexithymia should be examined in the future in combination with self-report and behavioral measures or observer assessments. Fourth, this study was limited to patients with chronic SCZ, and future studies should consider first-episode patients to eliminate the effects of disease course and drug treatment. Despite these limitations, this study was the first to examine the relationship between obesity and alexithymia in Chinese patients with chronic SCZ and to further investigate the heterogeneity of this relationship in obese and nonobese SCZ patients. This will help us to understand these features of SCZ and to better provide targeted interventions and treatments for different subtypes of SCZ patients in clinical practice.
To summarize, this study explored the correlation between alexithymia and clinical symptoms in obese and nonobese patients with chronic SCZ with obesity and nonobesity, and found some interesting results showing a significant association between difficulty in identifying feelings and positive symptoms in obese SCZ patients only, which may facilitate better targeted interventions and treatments. Specifically speaking, obese SCZ patients had greater positive symptoms and degree of alexithymia compared to nonobese SCZ patients, especially in difficulty identifying feelings and difficulty describing feelings. These findings implicated that obesity may be a potential an underlying factor in exacerbating positive symptoms and alexithymia in SCZ patients. Furthermore, alexithymia was strongly closely associated with positive symptoms and is only present in obese patients, which may have important theoretical and clinical implications for understanding the relationship between emotion dysregulation and psychopathological symptoms in SCZ patients. From a clinical perspective, in for SCZ patients with obesity, some emotion regulation strategies may be considered to improve the ability to identify feelings, and then indirectly improve positive symptoms. However, due to certain reported limitations, this study is preliminary, and future longitudinal studies using objective measures of alexithymia in patients with SCZ will help to further confirm our findings of this study.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Xiangyang Zhang. Data curation: Yang Tian. Formal analysis: Yang Tian. Funding acquisition: Xiangyang Zhang. Investigation: Yang Tian. Methodology: Xiangyang Zhang. Project administration: Xiangyang Zhang. Resources: Yang Tian. Software: Yang Tian. Supervision: Xiangyang Zhang. Validation: Xiangyang Zhang. Visualization: Yang Tian. Writing—original draft: Yang Tian. Writing—review & editing: Huixia Zhou, Dongmei Wang.
Funding Statement
This work was supported by CAS International Cooperation Research Program (153111KYSB20190004), CAS Pioneer Hundred Talents Program, and the CAS Key Lab of Mental Health.
Acknowledgements
The authors would like to thank the psychiatrists and nurses of the three hospitals for all of their hard work and great contributions toward the study.