Efficacy and Safety of Pulse Magnetic Therapy System in Insomnia Disorder: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial
Article information
Abstract
Objective
This study’s objective is to assess the efficacy and safety of Pulsed Magnetic Therapy System (PMTS) in improving insomnia disorder.
Methods
Participants with insomnia disorder were randomly assigned to receive either PMTS or sham treatment for four weeks (n= 153; PMTS: 76, sham: 77). Primary outcomes are the Insomnia Severity Index (ISI) scores at week 0 (baseline), 1, 2, 3, 4 (treatment), and 5 (follow-up). Secondary outcomes are the Pittsburgh Sleep Quality Index at baseline and week 4, and weekly sleep diary-derived values for sleep latency, sleep efficiency, real sleep time, waking after sleep onset, and sleep duration.
Results
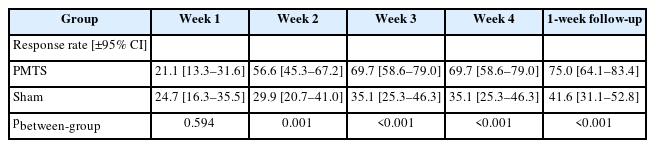
The ISI scores of the PMTS group and the sham group were 7.13±0.50, 11.07±0.51 at week 4, respectively. There was a significant group×time interaction for ISI (F3.214, 485.271=24.25, p<0.001, ηp2=0.138). Only the PMTS group experienced continuous improvement throughout the study; in contrast, the sham group only experienced a modest improvement after the first week of therapy. At the end of the treatment and one week after it, the response of the PMTS group were 69.7% (95% confidence interval [CI]: 58.6%–79.0%), 75.0% (95% CI: 64.1%–83.4%), respectively, which were higher than the response of the sham group (p<0.001). For each of the secondary outcomes, similar group×time interactions were discovered. The effects of the treatment persisted for at least a week.
Conclusion
PMTS is safe and effective in improving insomnia disorders.
INTRODUCTION
Insomnia disorder is defined by a set of subjective sleep symptoms, including difficulties initiating or maintaining sleep and the consequent daily dysfunction [1]. It is by far the most prevalent sleep disorder, affecting 3.9% to 22.1% of the general population [2,3]. It is commonly associated with other disorders such as depression, anxiety, diabetes, cardiovascular disease, neurodegenerative diseases, and cancer [4-6]. It has placed a heavy economic burden on individuals [7].
Practice recommendations on treating insomnia disorder are usually limited to psychological and pharmacological therapies [1,3]; however, both of them have limitations to some extent. Pharmacotherapies are associated with various side effects and could easily lead to dependence or misuse [8]. Psychological therapies, while being safer, do not response in all cases [9], and their application depends largely on the compliance of patients as well as the availability of professional therapists. Therefore, alternative therapies for insomnia disorders are still in demand.
Pulsed magnetic therapy is a noninvasive method of neural regulation that involves applying pulsed magnetic fields to living tissue to modulate cell activity [10]. It has been widely used to treat different diseases, such as osteoporosis [11], Parkinson’s syndrome [12], Alzheimer’s disease [13], oncology [14], pain [15], and so on. In general, applying pulsed magnetic fields of low frequency (≤1 Hz) to the head is thought to be inhibitory [16]. This suggests that low-frequency pulsed magnetic fields may have therapeutic potential to improve insomnia disorder: at a pathophysiological level, insomnia disorder is characterized by accumulated cortex hyperarousal processes, and might thereby be improved by low-frequency magnetic pulses [17,18]. One of the possible mechanisms is the regulation of brain-derived neurotropic factor and γ-aminobutyric acid [19,20].
The current study aims to assess the efficacy and safety of the Pulsed Magnetic Therapy System (PMTS), a portable therapeutic system developed to carry out low-frequency pulsed magnetic therapy during the process of falling asleep, for improving insomnia disorder.
METHODS
Study design and setting
This multi-center, double-blind, sham-controlled randomized clinical trial included a 1-week baseline observation, a 4-week PMTS or sham treatment, and a 1-week follow-up observation. The study design and protocol were approved by the ethics committees of three centers: the First Affiliated Hospital of Jinan University (Ethics Approval number: 2018-009), the Third Affiliated Hospital of Guangzhou Medical University (Ethics Approval number: 2019-009), and Shenzhen mental Hospital (Ethics Approval number: 2019-001-01). The protocol was registered at Chinese Clinical Trial Register (https://www.chictr.org.cn; identification number: CTR1900024627).
Participants
Sample size was calculated using nQueryTM Advanced (https://www.statsols.com/; Statsols, San Diego, CA, USA). The expected effect size was set as 0.5 according to previous studies that suggested a medium to high level of effect size [21,22]. With a two-tailed α=0.05, 1-β=0.80, and a drop-out rate=20%, a sample size of 77 participants per group (154 in total) was necessary.
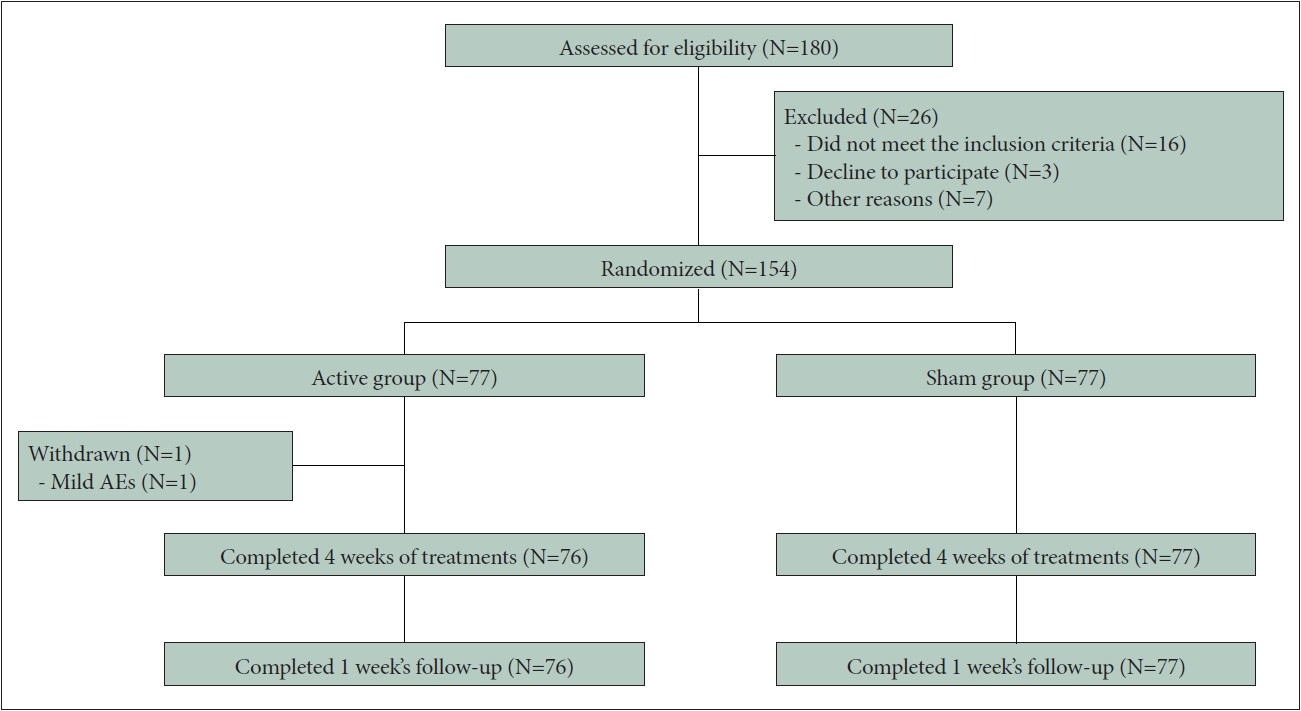
Participants were enrolled at the three research centers or via social media advertisements. Participants predetermined by their clinicians as being potentially suitable and having interest in the current study underwent a set of screening, including a structural clinical interview, diagnostic scales (Insomnia Severity Index [ISI], Pittsburgh Sleep Quality Index [PSQI], Generalized Anxiety Disorder-7 [GAD-7], and Patient Health Questionnaire-9 [PHQ-9]), blood pressure and heart rate measurements, and a single-night at-lab polysomnography (PSG). A total of 153 participants were included in the final sample (Figure 1). All participants provided written informed consent.
The inclusion criteria were: 1) meeting the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition [23] diagnostic criteria for insomnia disorder (F51.01); 2) being aged 18 to 65 years; and 3) signing the informed consent form.
The exclusion criteria were: 1) insomnia associated with any physical or mental disorders which was caused by substance use; jetlag or work shifts; 2) untreated obstructive sleep apnea syndrome (apnea–hypopnea index >15/h) or periodic limb movement of sleep (Periodic Limb Movement of Sleep Index >15/h), which was determined by overnight PSG, or other severe physical health issues (e.g., cancer, AIDS, cardiovascular, and liver and renal dysfunction); 3) moderate to severe anxiety or depression (GAD-7 >9, PHQ-9 >9); 4) employments that are not suitable for the test; 5) pregnancy; 6) history of epilepsy; 7) current or past neural modulation treatment; 8) implanted with metal or magnets; 9) participated in other clinical studies in 3 months; and 10) other issues determined by the researchers.
Enrolled participants were randomized 1:1 to the PMTS or sham group according to a predetermined random-number table-generated allocation sequence. This randomization sequence was generated by an independent third-party using SAS 9.4 (www.sas.com; SAS Institute, Cary, NC, USA), and was concealed from participants, investigators, and statisticians.
Interventions
PMTS version 1.0 (BestCare & SuMian BioTech Co., Ltd., Dongguan, China) is a portable therapeutic system designed for noninvasive sleep improvement. It consists of a portable pulsed magnetic field generator positioned under the head as the participant goes to bed, and a wrist sleep monitor tracking real time sleep stage. It is designed to generate pulsed magnetic fields of 1 Hz as the participant goes to sleep. The generation of magnetic pulses is controlled by algorithm according to the sleep-stage data, so that the stimulation is stopped after falling asleep.
In the current study, the PMTS group received active PMTS intervention every night over the 4 weeks of treatment. The sham group received fake intervention, in which all the procedures were the same except that the generators did not actually output pulsed magnetic fields. To ensure that the sham group was blind to their grouping, the sham generators emitted mild heat to simulate the working mode of the active generators.
Outcome measurements
The primary outcome was the changes in total score of the ISI, a 7-item symptomatic measure of insomnia that has good validity, reliability, and sensitivity to short-term insomnia changes, assessed at the end of each week. Usually, a total score of 7 or less indicates no clinically significant (insomnia) [24], while according to Chung et al. [25], 9 is suggested to be a better cutoff point for Chinese populations. Therefore, the current study defined a total ISI score of ≤9 as response. The response rate was calculated by dividing the number of responses by the number of participants.
Secondary outcomes included PSQI scores at the end of baseline and week 4, and weekly sleep diary-derived values for sleep latency, sleep efficiency, actual sleep time, and wake after sleep onset (WASO).
Statistical methods
Data were analyzed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA). A multiple imputation approach was used to impute missing values. General linear models were used to test group×time interactions and post-hoc analysis between-group and between-time effects for the ISI, PSQI, and sleep diary-derived outcomes. Generalized estimating equations were conducted for response rates over time. A Pearson’s χ2 test or Fisher’s exact test was conducted for baseline characteristics and adverse events (AEs). All tests were two-sided, with a test level of α=0.05.
RESULTS
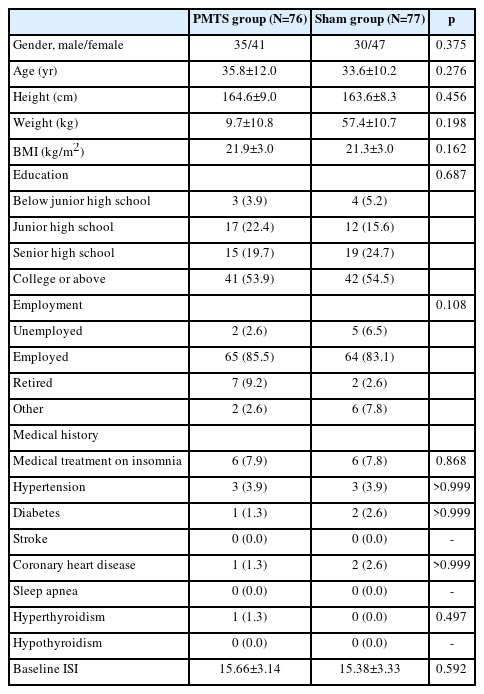
A total of 180 participants were screened for eligibility between July 2019 and August 2020; of these, 26 subjects failed to be screened, 154 were enrolled. One subject withdrew from the study due to inconvenient transportation before treatment after signing the informed consent form and 153 of those participants were included in the final sample (Figure 1). Table 1 lists the baseline characteristics; no significant group difference was found for any of these parameters.
Primary outcomes
Results of the primary outcome, the ISI scores, were presented in Figure 2. The ISI scores of the PMTS group and the sham group were 7.13±0.50, 11.07±0.51 at week 4, respectively. There was a significant group×time interaction (F3.214, 485.271=24.25, p<0.001, ηp2=0.138). Simple effect analysis revealed that the PMTS group improved weekly during the whole study (changes from baseline: Cohen’s d=1.07, 1.69, 1.92, 2.21, and 2.38). The sham group also improved significantly within the first week (changes from baseline: Cohen’s d=0.95), but then only improved very mildly afterward (changes from baseline: Cohen’s d=0.96, 1.07, 1.12, and 1.21). Analysis of group effect indicated that the PMTS group began to overperform the sham group since the 2nd week (post-hoc between-group p=0.592, 0.652, <0.001, <0.001, and <0.001; Cohen’s d=0.09, 0.07, 0.59, 0.73, 0.86, and 0.98 at the end of each week).
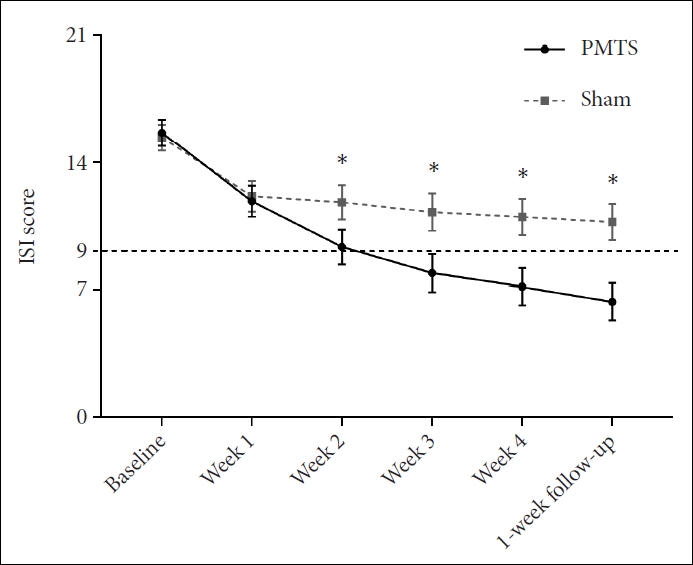
The ISI scores of the PMTS group and the sham group. An ISI score ≤9 indicated no clinically significant insomnia disorders. Error bars indicated ±95% CI. *pbetween-group<0.05. ISI, Insomnia Severity Index; PMTS, Pulsed Magnetic Therapy System; CI, confidence interval.
In addition, an ISI ≤9 was defined as not having clinically significant insomnia disorder, or as response (Table 2). Results showed a similar interactive between group and time (χ2=17.69, p=0.003). The PMTS group had outperformed the sham group since week 2, while nearly 70% of the participants who received PMTS treatment did not show clinically significant insomnia disorder at the end of the intervention.
Secondary outcomes
A significant group×time interaction (F1,151=18.05, p<0.001, ηp2=0.107) was found on PSQI scores (Figure 3A). Specifically, both groups improved after intervention compared to baseline (PMTS group: Cohen’s d=1.58; sham group: Cohen’s d=0.80), while the PMTS group experienced a greater improvement (between-group Cohen’s d=0.15, 0.58 for baseline and week 4, respectively).

PSQI scores and weekly sleep diary-derived value of the PMTS group and the sham group. A: PSQI score. B: Sleep latency. C: Sleep efficiency. D: Actual sleep time. E: Wake after sleep onset. Error bars indicated ±95% CI. *pbetween-group<0.05; **pbetween-group<0.01; ***pbetween-group<0.001. PMTS, Pulsed Magnetic Therapy System; PSQI, Pittsburgh Sleep Quality Index; CI, confidence interval.
Weaker but still significant group×time interactions were found for all the diary-derived indexes (Figure 3B-E): sleep latency (F3.822, 577.116=5.01, p=0.001, ηp2=0.032), actual sleep time (F3.851, 581.466=3.60, p=0.007, ηp2=0.023), sleep efficiency (F3.937, 594.482=4.28, p=0.002, ηp2=0.028), and WASO (F3.312, 500.085=2.94, p=0.028, ηp2=0.019).
AEs
Participant-reported AEs were compared between groups (Table 3). There were 26 and 42 cases of AEs related to the research medical device in the experimental group and the control group, respectively. Among them, 67 cases were mild, 1 case was moderate, and none of them were severe in severity. There was no significant difference in the incidence of AEs between groups (p=0.052).
DISCUSSION
This multi-center, double-blind, sham-controlled, and randomized clinical trial investigated the efficacy and safety of a portable PMTS among a sample of the adult population with insomnia disorder, ranging in age from 18 to 65 years old. Participants who received PMTS treatment continuously experienced significant improvements in various outcomes, including ISI, PSQI, diary-derived sleep latency, and actual sleep time, during 4 weeks of treatment and 1 week of followup, while the sham group only improved slightly after the first week. Nearly 70% of the participants at the end of the treatment and 75% after 1 week’s follow-up in the PMTS group no longer exhibited clinically significant insomnia symptoms (ISI ≥9), almost twice as much as in the sham group. Analysis of AEs further revealed that PMTS was safe to users. Together, these results revealed a good efficacy and safety of PMTS on insomnia disorder.
The sham group showed a comparable improvement to the PMTS group only in the first week, which indicated a significant placebo effect for it. Similar placebo effects had been consistently found for pulsed magnetic therapy on treating insomnia disorders [21,22]. This placebo effect highlighted the necessity of the randomized controlled design of the current study. What is more, it may also explain the higher rate of headaches or dizziness in the sham group. Headaches could be due to stress-induced hyperarousal [26]. While an unfamiliar medical instrument may elicit negative affect and stress among all participants, PMTS neutralized these arousals and thus reduced headache incidences in the PMTS group. PMTS may have the potential to be a sleep-aid tool for other stress-induced disorders, e.g., depression, anxiety, and chronic pain. Future studies among these populations are still needed.
The current findings have several implications on nonpharmacological management for insomnia disorder. The efficacy is consistent with previous studies that documented the efficacy of pulsed magnetic therapy to improve insomnia disorder [27,28].
However, considering that existing pulsed magnetic therapies, such as repetitive transcranial magnetic stimulation, usually require generating high intensity fields (>1 T) and identifying targets and motor evoked potential before treatment, it is technically impossible to be applied at home so far. On the contrary, PMTS managed to be portable because it generated a much weaker pulsed magnetic field in the head globally. Our findings proposed that PMTS should be a good choice for at-home at-night insomnia intervention.
Limitations and generalizations
The current findings must be considered in light of several issues. First, although the two groups were highly comparable in ISI and PSQI at baseline, we did find a tendency that dairy-derived indexes were worse for the PMTS group. This may cast some doubt on the homogeneity of our sample; however, the significant find of the group×time interactions strongly supported the efficacy of the treatment. Second, various populations, such as the elderly, children and adolescents, pregnant women, and patients with other physical or mental disorders, were not included in the current study. Although studies concerning pulsed magnetic therapy have generally found a good tolerance for such techniques among different populations [29], considering the need for at-home usage, it must be highly cautious to apply PMTS to these populations. Third, considering the majority (93.5%) of the participants with mild to moderate insomnia disorder at baseline, the conclusions of current study should not be directly drawn on severe insomnia disorder, which is found to be closely associated with cortical hyperarousal [18,30]. PMTS is therefore expected to be more efficient than psychological treatments for severe insomnia disorder [30], but still needs further research. Fourthly, objective outcome was not included like PSG or actigraphy and follow-up duration was too short to evaluate the durability or sustainability of efficacy of PMTS.
Conclusion
The current study provides compelling evidence that a portable pulsed magnetic therapy system, PMTS, is of good efficacy and safety among adults with mild to moderate insomnia disorder. The positive effect of PMTS on insomnia patients could last for at least 1 week. Considering its portability and user friendliness, PMTS has great promise as an at-home atnight treatment for insomnia disorder.
Notes
Availability of Data and Material
After this work is published, any anonymized data collected will be available for sharing.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jiwu Liao, Jiyang Pan. Data curation: Sisi Wang, Shanlian Zhang. Formal analysis: Borong Zhou, Tianhua Zhu. Funding acquisition: Wei Liang. Investigation: Jiwu Liao, Ping Ma, Min Lin, Weisen Lin, Aibing Fu, Lu Xing, Wumei Li. Methodology: Congrui Li, Yin Cui, Jiajia Hu, Yuanyi Qin, Yanhua Deng, Yunhong Qu. Project administration: Xiaotao Zhang, Hongyao Li, Aibing Fu, Xinping Yao. Supervision: Borong Zhou, Fei Feng, Jiyang Pan. Writing—original draft: Jiwu Liao. Writing—review & editing: Jiwu Liao, Yanhua Deng, Guimei Zhang.
Funding Statement
This study is sponsored by BestCare & SuMian BioTech Co., Ltd., who also provided the PMTS. The sponsor had no role in the data analysis, interpretation, or scientific writing of this study. This manuscript solely reflects the views of the authors.
Acknowledgements
We thank the researchers and participants for their time and thoughtful responses during interviews.