Characteristics of Cognitive Function Changes and Related Factors in Individuals With Cognitive Impairment During the Pandemic of COVID-19: A Retrospective Chart Review Study
Article information
Abstract
Objective
This study aimed to explore the characteristics and factors related to changes in cognitive function in vulnerable individuals with cognitive impairment during the coronavirus disease 2019 (COVID-19) pandemic.
Methods
Among patients who visited a local university hospital with subjective cognitive complaints, those who had been tested for cognitive function at least once after the onset of COVID-19 and tested regularly at least three times within the last 5 years were included (1st, the initial screening; 2nd, the test immediately before the COVID-19 pandemic; 3rd, the most recent test after the pandemic). Finally, 108 patients were included in this study. They were divided into groups according to whether the Clinical Dementia Rating (CDR) was maintained/improved and deteriorated. We investigated the characteristics of the changes in cognitive function and related factors during COVID-19.
Results
When comparing CDR changes before and after COVID-19, there was no significant difference between the two groups (p=0.317). Alternatively, the main effect of the time when the test was conducted was significant (p<0.001). There was also a significant difference in the interaction between the groups and time. When the effect of the interaction was analyzed, the CDR score of the maintained/improved group significantly decreased before COVID-19 (1st–2nd) (p=0.045). After COVID-19 (2nd–3rd), the CDR score of the deteriorated group was significantly higher than that of the maintained/improved group (p<0.001). Mini-Mental State Examination recall memory and changes in activity during COVID-19 were significantly associated with CDR deterioration.
Conclusion
Memory dysfunction and decreased activity during the COVID-19 pandemic are strongly related to the deterioration of cognitive impairment.
INTRODUCTION
Decreased cognitive function is a common feature of aging and is associated with poor quality of life, a lack of functional independence and mortality [1,2]. Cognitive decline is a hallmark of Alzheimer’s disease and other dementias, often indicating their onset [3,4]. Older adults suffering from cognitive decline are more likely to demand continuous care from their families and society, which, in turn, increases the burden on family members and social insurance funds. Dementia has higher health and social care costs ($16.2 bn) than cancer ($6.8 bn) and chronic heart disease ($3.4 bn) combined [5]. The cost of dementia globally reached $1 trillion in 2018 and would double to $2 trillion by 2030 [6].
Therefore, effective population-based strategies need to be established to prevent or delay cognitive decline in the older population. Previous studies have shown that cognitive decline is associated with sociodemographic factors [7], lifestyle [8,9], neuropsychiatric problems [10], chronic illness [11,12], and social isolation [13,14]. A cognitively and socially enriched lifestyle is important for older adults, especially to maintain their level of independence, mental health, and well-being [15,16].
The World Health Organization (WHO) has warned that vulnerable groups, such as older people and individuals with underlying health conditions, are facing the most serious threats and challenges to mental and psychosocial health [17]. Older adults with impaired cognition are at high risk of being infected with coronavirus disease 2019 (COVID-19) [18], which increases the risk of disease-related morbidity and mortality [19]. The pandemic also intensifies their vulnerability because of the adverse effects of increased isolation [20], lack of physical exercise [21,22], reduced social involvement [22,23], and intentional cessation of activities [21].
After the emergence of COVID-19 in South Korea, a coronavirus pandemic rapidly worsened in February 2020 with a specific religious group gathering in the Daegu area. Accordingly, the Korean government implemented “social distancing” as a recommendation to prevent the virus from spreading. Social distancing can be implemented in many ways, with its aim being to keep people apart from each other to reduce contact rates. It included restrictions on private gatherings, events and assemblies, observation of personal hygiene, control of unnecessary outings, and reinforcement of quarantine management of multi-use facilities. Social distancing has decreased activities and affected most people’s daily lives, who remain confined to their homes [24,25].
Accessing social support systems and participating in social activities are key to supporting the well-being of people with cognitive impairment. However, the advent of the COVID-19 era will cause activity limitations and result in multiple clinical changes in individuals with cognitive impairment. The present study aimed to investigate the characteristics of cognitive function changes in individuals with cognitive impairment who are vulnerable to social distancing and loss of activities during the pandemic, and to explore the related factors.
METHODS
Participants
All participants were outpatients or inpatients with subjective cognitive complaints who visited Yeungnam University Hospital between January 2017 and February 2021. A flowchart of the participants is shown in Figure 1. A total of 1,405 participants underwent initial evaluation during this period. Those who had been tested at least once since the outbreak of COVID-19 and underwent assessment of cognitive function and social activity level three times or more regularly within the last 5 years were included. The initial screening was classified as the 1st trial, the test immediately before the COVID-19 pandemic outbreak as the 2nd trial, and the most recent test after the outbreak as the 3rd trial. Participants who did not undergo the assessment regularly and those aged under 60 years who had cognitive dysfunction due to organic brain damage were excluded (Figure 1).
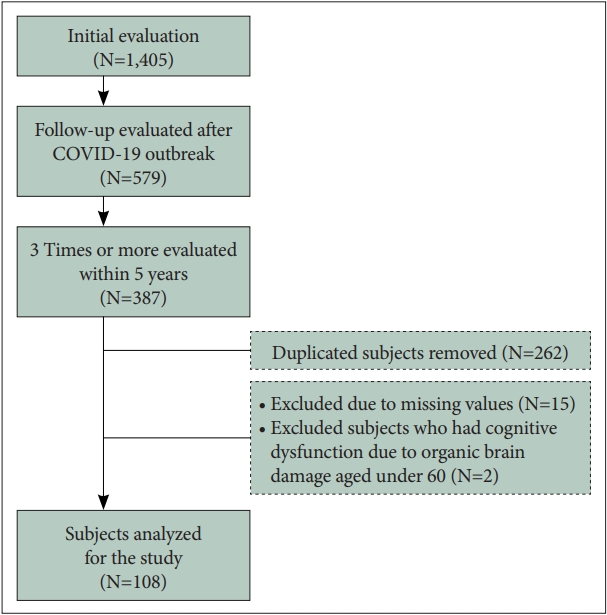
Summary of the evaluation process for all participants. The 1st, initial screening test; 2nd, test immediately before the coronavirus disease 2019 (COVID-19) pandemic outbreak; 3rd, most recent test after the COVID-19 outbreak.
Finally, 108 patients were included in this study. They were divided into groups according to whether the Clinical Dementia Rating (CDR) was maintained/improved and deteriorated. The differences between CDR changes before and after COVID-19 (2nd trial vs. 3rd trial) were compared. Additionally, we analyzed the interaction between the groups (CDR maintained/improved and deteriorated groups) and period (before and after COVID-19). This study was approved by the Institute Review Board of Yeungnam University Medical Center (YUMC 2021-06-039).
Procedure and measures
Assessment of cognitive function
The evaluation results from the participants were collected by a psychiatrist and trained psychology graduates at baseline and follow-up.
The Korean version of the Mini-Mental State Examination
The Mini-Mental State Examination (MMSE) was used to assess cognitive function in the study participants. In South Korea, the Korean version of the MMSE (K-MMSE) has been developed and is widely used to evaluate cognitive impairment [26]. For the purpose of this study, 11 subtests comprising the MMSE and global MMSE scores were considered independently. The MMSE comprises 11 major items: temporal orientation (5 points), spatial orientation (5 points), immediate memory (3 points), attention/concentration (5 points), delayed recall (3 points), naming (2 points), verbal repetition (1 point), verbal comprehension (3 points), writing (1 point), reading a sentence (1 point), and visual construction (pentagon copying, 1 point). Pentagon copying consisted of the subject drawing two intersecting pentagons. The MMSE has a maximum score of 30, with five different domains of cognition analyzed: 1) orientation, contributing a maximum of 10 points; 2) memory, contributing a maximum of 6 points; 3) attention and calculation, as a measure of working memory, contributing a maximum of 5 points; 4) language, contributing a maximum of 8 points; and 5) design copying, contributing a maximum of 1 point. The K-MMSE score is influenced by education and age; therefore, a single score was not used in the diagnosis of dementia. The score was standardized by correcting for age and educational background [27].
CDR
The CDR evaluates three domains of cognition (memory, orientation, and judgment/problem-solving) and three domains of function (community affairs, home/hobbies, and personal care), and is a semi-structured interview involving a subject and an informant. The six CDR domains were rated from 0 (no impairment) to 5 (most severe impairment), and two primary CDR scores were derived. From the six individual category ratings, or box scores, the CDR-global score (GS) was established by clinical scoring rules. The scores for the six domains were summed to obtain a CDR-sum of boxes (SOB) score ranging from 0 to 30, with a higher score indicating more severe impairment and a higher likelihood of dementia. The CDR demonstrates good reliability [28,29] and has been validated against neuropathologic finding [30-32].
Assessment of social activities
An activity-level scale was used to assess daily function and social activity, in addition to cognitive function. Various scales for evaluating the level of activity in the general population have been devised and are currently being used. Activity-level measures such as Quick Physical Activity Rating, Saltin-Grimby Physical Activity Level Scale, Physical Activity Scale for the Elderly are used in research [33-35]. However, the above scales are helpful for the functionally normal population, and there is a limit to their application to the older adults with cognitive dysfunction who have limited activity due to deterioration of physical and cognitive functions or those who maintain minimal daily functions. There is a significant difference in the amount of activity and sedentary time of the elderly with dementia compared to the general population [36,37].
Thus, in this study, we created a new scale that can be easily and quickly applied, although its validity and reliability have not yet been verified. Activity level was based on the patients’ daily activity function, social activity participation, interpersonal activity, and cognitive function intervention. We classified the patients based on their medical records and the level of social activity domains on the CDR scale, Korean-instrumental activities of daily living, and Barthel activities of daily living. The activity domains were rated on a scale of 1–5 points (1=voluntary and active, 2=not active but voluntarily maintained, 3=limited or only maintained with assistance, 4=extremely limited or minimal with assistance, and 5=unable with or without assistance).
Sociodemographic characteristics as covariates
We reviewed the patients’ charts with complete medical records, including age, sex, education year, cognitive intervention, and geriatric depression scale (GDS) score. Cognitive interventions included cognitive training, cognitive stimulation, and cognitive rehabilitation at home or in community care.
Analyses
The interaction effect between the groups (CDR maintained/improved and deteriorated groups) and period was analyzed using survival analysis. Variables were analyzed using the Cox proportional hazards model for survival analysis. These models applied the step-forward method and tested whether specific variables (i.e., activity level scores, neuropsychological test scores, and demographic factors) affected the time leading to a specific outcome with CDR score deterioration. We performed a two-way ANOVA to compare how the changes in CDR differed according to two independent variables: the CDR-GS deteriorated group and clinical assessments before and after the COVID-19 pandemic. Independent and paired t-tests were conducted to understand the interaction effect in detail. Statistical analyses were performed using IBM SPSS version 22.0 for Windows (IBM Corp., Armonk, NY, USA). For all tests, the level of significance was set at p<0.05.
RESULTS
Demographic characteristics
This study included 108 participants (40 male and 68 female) who had their cognitive function and level of activity assessed three times or more within 5 years, including those performed before and after the COVID-19 pandemic. The mean and standard deviation of the participants’ age and years of education were 70.78±10.46 years and 7.32±5.29 years, respectively. Twenty-three participants (21.3%) continued cognitive intervention, while 85 (78.1%) withdrew from or did not receive intervention (Table 1).
Changes in CDR and correlation with the time when tests were implemented
When the maintained/improved (n=68) and deteriorated (n=40) groups were classified based on the changes in the CDR-GS during the study period, there was no significant difference observed between the sociodemographic variables, and depression scale scores between the two groups (Table 2).
In this study, we investigated the CDR changes by dividing the examination period of each patient. The total number of trials in the CDR maintained/improved and deteriorated groups were 5.13 and 5.30, respectively (Table 3). The initial screening was classified as the 1st trial, the test immediately before the COVID-19 pandemic outbreak as the 2nd trial, and the most recent test after the outbreak as the 3rd trial. Table 3 shows the K-MMSE mean values, CDR-GS distribution, and CDR-SOB distribution for each trial.
In the administered period, the average trial interval between the 1st trial and the 2nd trial was significantly shorter in the CDR-deteriorated group than in the maintained/improved group (p<0.01). In both CDR-GS and SOB, the range of the CDR distribution in the deteriorated group was significantly different from that in the maintained/improved group in the 3rd trial (Table 3).
When comparing CDR changes before and after COVID-19, there was no significant difference between the two groups (p=0.317). Alternatively, the main effect of the time when the test was conducted was significant (p<0.001). There was also a significant difference in the interaction between the groups and time (p<0.001) (Table 4). There was no significant difference between the two tests before the COVID-19 outbreak. However, tests immediately before COVID-19 (2nd trial) and after COVID-19 (3rd trial) reflected results with a significant difference (Figure 2).

Changes in participants of CDR maintained/improved group and deteriorated group. CDR–global scores significantly increased between 2nd trial and 3rd trial in the CDR deteriorated group. CDR, clinical dementia rating; COVID-19, coronavirus disease 2019; GS, global score.
When the effect of the interaction was analyzed, the CDR score of the maintained/improved group significantly decreased before COVID-19 (1st–2nd) (p=0.045). After COVID-19 (2nd–3rd), the CDR score of the deteriorated group was significantly higher than that of the maintained/improved group (p<0.001) (Table 5). Therefore, we attempted to identify the factors related to the COVID-19 pandemic that might affect dementia deterioration.
The variables related to cognitive deterioration
Survival analysis was performed as a CDR deterioration event. MMSE recall memory and changes in activity in COVID-19 were significantly associated with CDR deterioration (Table 6). Therefore, a higher memory recall score was associated with a lower risk of CDR deterioration, and those who maintained their activity level during the COVID-19 pandemic had a lower risk than those who did not. Similar results were observed when evaluated for the effect of time.
The effect of activity changes due to COVID-19 on dementia deterioration
Furthermore, the effect of changes in activity on the deterioration of dementia symptoms was analyzed (Table 7). Cox regression analysis of the changes in activity showed the same associations between CDR and activity. Participants with decreased activity showed a shorter time to deteriorating CDR-GS and CDR-SOB scores than those with maintained activity (Figures 3 and 4).
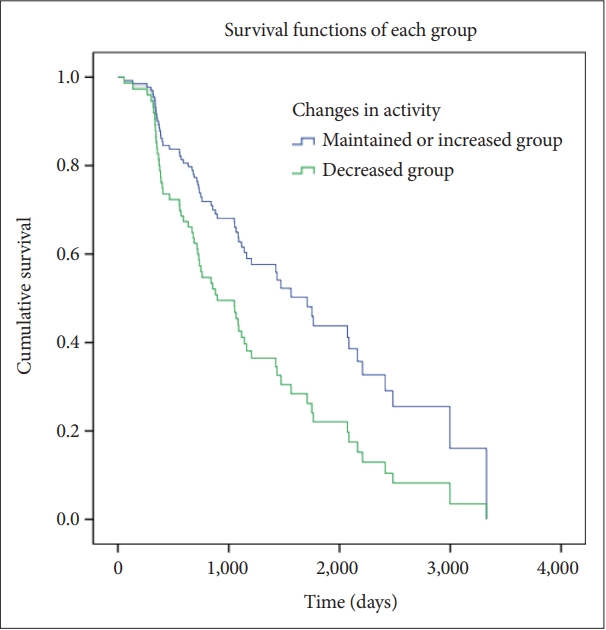
Correlations between the decrease in the activity and the degree of deterioration in clinical dementia rating–global score.
DISCUSSION
We investigated the characteristics of changes in cognitive function and related factors in individuals with cognitive impairment during the COVID-19 pandemic in a specific metropolitan city. The COVID-19 pandemic has had a significant impact on cognitive function in individuals with cognitive impairment, particularly subjects with memory recall impairment or decreased activity at high risk.
The COVID-19 era has certainly brought about several drastic changes in the global population [25]. Among them, it has resulted in significant clinical changes in individuals with cognitive impairment [22]. Social distancing measures are a way to protect older people from COVID-19, but consistent results reported during the pandemic are that they are associated with a higher rate of neuropsychiatric symptoms due to reduced physical and social activity and isolation [24,41]. In addition, a high rate of abandoned previous daily activities, cognitive worsening reported by relatives/caretakers, delirium episodes, and increased incidence of falls have been reported in COVID-19 [23], which are known to be associated with worsening dementia [42-45]. It can also increase the risk of social burden, management difficulties, medication prescriptions, and other complications that accompany cognitive decline.
Although the clinical symptoms of cognitive disorders persist and worsen over time due to the course of the disease, this study showed that cognitive decline significantly accelerated after the COVID-19 pandemic outbreak compared with previous evaluations in the same participants before the COVID-19 outbreak. There was no difference in demographic factors between the participants with and without cognitive deterioration. There was also no significant difference in GDS scores, which could affect cognitive decline and needs to be differentiated.
In the administered period, the average trial interval between the initial screening (1st trial) and the test immediately before the COVID-19 pandemic outbreak (2nd trial) was significantly shorter in the CDR-deteriorated group than in the maintained/improved group (p<0.01). In the case of the deteriorated group, various factors in the clinical setting, such as anxiety or requests of patients and caregivers, and concerns of clinicians led to more frequent examinations.
When comparing CDR changes before and after COVID-19, there was no difference between the two tests before COVID-19 (p=0.433), but tests immediately before COVID-19 (2nd trial) and after COVID-19 (3rd trial) showed a significant difference (p<0.001). The interaction effect of the group and time of test implemented confirmed a significant decrease in the CDR between the 1st and 2nd tests of the maintained/improved group and a significant increase in the CDR between the 2nd and 3rd tests of the deteriorated group. These results suggest that the outbreak of the COVID-19 pandemic is directly or indirectly related to the deterioration of cognitive function in individuals with cognitive impairment.
Considering the variables affecting CDR deterioration revealed through further analysis, it can be noted that environmental factors, that is, changes in activities due to COVID-19, may be one of the related factors. Previous studies have shown that memory impairment [46,47]. Alzheimer disease diagnosis [48], basic activities of daily living dependencies [49] and other psychological symptoms [50-52] are known factors related to the rapid progression of dementia. Identifying individuals with rapid cognitive decline and its affecting factors is important because it has worse functional outcomes [53] and is associated with higher mortality [54,55]. The fact that COVID-19 contributed to changes in activity has been consistently reported in previous studies [56,57]. A notable decline in activity was confirmed with quarantine based on social distancing [18,25]. Our study revealed an association between changes in an individual’s activity level and cognitive change due to environmental changes caused by large-scale infectious diseases.
In particular, we can consider the limitations of several approaches for managing cognitively impaired individuals [20,58]. The Dementia National Responsibility System in our country provides various services for individuals with cognitive impairment. However, owing to the spread of COVID-19, the operation of the program has been suspended or reduced, and the number of patients using these services has also decreased. In fact, compared to 2019, the number of dementia screening tests decreased by 56.4% from 1,803,474 to 786,389 in 2020, and the number of people who use the intervention centers for dementia also decreased by 16.8% from 8,491 to 7,065. In light of the fact that the rate of cognitive function deterioration was slower in the group that continued activity in this study, changes in cognitive intervention treatment and program operation may have influenced on the cognitive changes of elderly subjects.
Decreased recall memory is also related to the deterioration of clinical cognitive impairment. Memory function is the main ability to maintain daily functions [59,60] and memory difficulties adversely affect the quality of life and the independence of older adults [60,61]. In particular, decreased recall memory is highly correlated with impairment of hippocampal consolidation function [62,63]. It is known that the early damaged brain region in Alzheimer’s disease is the medial temporal region, including the hippocampus [64,65], and studies using structural and functional brain imaging have shown that volume differences and inactivation in theses area in Alzheimer’s disease compared to the general population [66-69]. The decline in recall memory function is not only a factor related to dementia progression in mild cognitive impairment [70] but also related to rapid cognitive decline in dementia patients [46,47]. In this study, there was a significant impairment in recall memory function in the CDR-deteriorated group compared to the maintained/improved group. This demonstrates the importance of proper memory function evaluation during the disease course, as well as the usefulness and potential for developing a management model for cognitive decline.
There are several limitations in this study. As this study was conducted on patients who continuously visited the hospital in a specific area, it could not be possible that the results represent the general population. And since this study is a retrospective study, it has limitations inevitable. Nonetheless, we explored the effects of COVID-19 through long-term tracking of patients with cognitive impairment, which could be verified through a comprehensive neuropsychological test. To make this more clearer, we only extracted subjects who had 3 or more test results, including before and after COVID-19. Since this includes two tests before COVID-19, we’ve been trying to determine the impact of COVID-19. Additionally, it is suggested that a comprehensive and meticulous evaluation can help identify the deterioration of cognitive function and take preventive measures in individuals complaining their cognitive function. In addition, although it is not a validated scale, we tried to measure the activity level. It showed that interventions such as maintaining physical activity, performing daily activities, participating in social work, and cognitive intervention are necessary for individuals with cognitive impairment during even the pandemic. Based on these results, novel methods and alternatives are needed for the management and control of cognitive impairment [71,72]. Due to the pandemic as well as environmental changes and media development, there has been an increasing interest in the importance and effectiveness of non-pharmacological interventions [73-75] that can be delivered at home [76-78], or using small portable devices [79,80]. Therefore, further studies are needed to recognize the link between factors leading to changes in physical and social activity that may help in the prevention and management of cognitive impairment. In other words, the findings of this study showed that the concerns raised about this group at risk during and after the COVID-19 pandemic are justified, and that individuals with cognitive impairment require more attention.
In conclusions, this study found a significant difference in cognitive function changes before and after the COVID-19 pandemic. Our results suggest that memory dysfunction and decreased activity during the COVID-19 period are strongly related to the deterioration of cognitive impairment.
Notes
Availability of Data and Material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jin-Hui Choi, Hye-Geum Kim. Data curation: Jin-Hui Choi, Ji Yean Kim, Hyun-Seok Jeong, Younggyo Kim, Hye-Geum Kim. Formal analysis: Jin-Hui Choi, Ji Yean Kim, Hyun-Seok Jeong, Younggyo Kim, Hye-Geum Kim. Funding acquisition: Hye-Geum Kim. Investigation: Jin-Hui Choi, Hye-Geum Kim. Methodology: Jin-Hui Choi, Hye-Geum Kim. Project administration: Hye-Geum Kim. Resources: Jin-Hui Choi, Bon-Hoon Koo, Wan-Seok Seo, Eun-Jin Cheon, Hye-Geum Kim. Supervision: Jin-Hui Choi, Bon-Hoon Koo, Wan-Seok Seo, Eun-Jin Cheon, Hyung-Mo Sung, Hye-Geum Kim. Validation: Jin-Hui Choi, Bon-Hoon Koo, Wan-Seok Seo, Eun-Jin Cheon, Hyung-Mo Sung, Hye-Geum Kim. Visualization: Jin-Hui Choi, Hye-Geum Kim. Writing—original draft: Jin-Hui Choi. Writing—review & editing: Jin-Hui Choi, Hye-Geum Kim.
Funding Statement
This study was supported by the 2020 Yeungnam University Medical Center COVID-19 Research Grant.








