 |
 |
- Search
| Psychiatry Investig > Volume 20(2); 2023 > Article |
|
Abstract
Objective
Methods
Results
Notes
Availability of Data and Material
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jin-Hui Choi, Hye-Geum Kim. Data curation: Jin-Hui Choi, Ji Yean Kim, Hyun-Seok Jeong, Younggyo Kim, Hye-Geum Kim. Formal analysis: Jin-Hui Choi, Ji Yean Kim, Hyun-Seok Jeong, Younggyo Kim, Hye-Geum Kim. Funding acquisition: Hye-Geum Kim. Investigation: Jin-Hui Choi, Hye-Geum Kim. Methodology: Jin-Hui Choi, Hye-Geum Kim. Project administration: Hye-Geum Kim. Resources: Jin-Hui Choi, Bon-Hoon Koo, Wan-Seok Seo, Eun-Jin Cheon, Hye-Geum Kim. Supervision: Jin-Hui Choi, Bon-Hoon Koo, Wan-Seok Seo, Eun-Jin Cheon, Hyung-Mo Sung, Hye-Geum Kim. Validation: Jin-Hui Choi, Bon-Hoon Koo, Wan-Seok Seo, Eun-Jin Cheon, Hyung-Mo Sung, Hye-Geum Kim. Visualization: Jin-Hui Choi, Hye-Geum Kim. Writing—original draft: Jin-Hui Choi. Writing—review & editing: Jin-Hui Choi, Hye-Geum Kim.
Funding Statement
This study was supported by the 2020 Yeungnam University Medical Center COVID-19 Research Grant.
Figure 1.
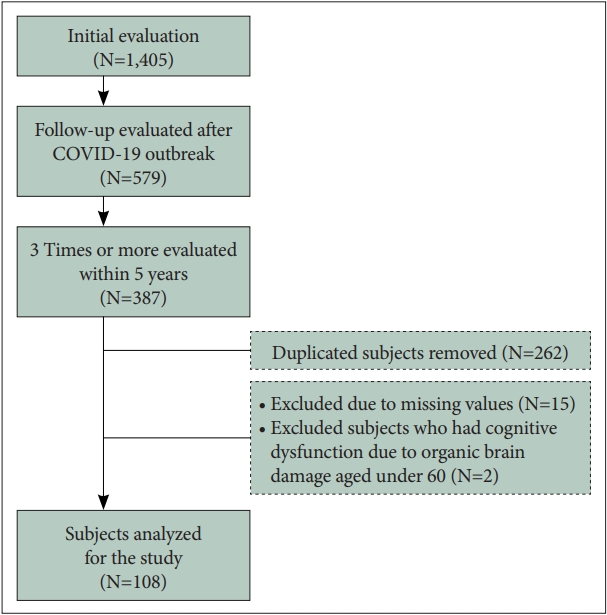
Figure 2.

Figure 3.
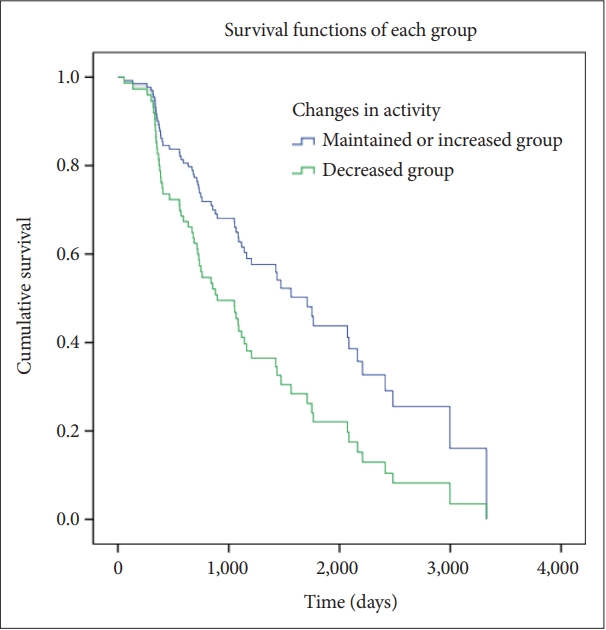
Figure 4.

Table 1.
| Characteristic | Value |
|---|---|
| Age (yr) | 70.78±10.46 |
| Gender | |
| Male | 40 (37.0) |
| Female | 68 (63.0) |
| Education (yr) | 7.32±5.29 |
| Cognitive intervention | |
| Yes | 23 (21.3) |
| No | 85 (78.7) |
Table 2.
Table 3.
| Maintained/improved (N=68) | Deteriorated (N=40) | t or χ2 | |
|---|---|---|---|
| Total number of trials | 5.13±2.12 | 5.30±2.67 | -0.36 |
| Administered period | |||
| 1st-2nd (days) | 488.78±188.72 | 373.08±185.83 | 3.107† |
| 2nd-3rd (days) | 396.09±129.36 | 416.83±153.39 | -0.750 |
| CDR-GS | |||
| 1st | 7.273 | ||
| 0 | 0 (0.00) | 3 (7.50) | |
| 0.5 | 4 (5.88) | 5 (12.50) | |
| 1 | 38 (55.88) | 22 (55.00) | |
| 2 | 20 (29.41) | 7 (17.50) | |
| 3 | 6 (8.82) | 3 (7.50) | |
| 4 | 0 (0.00) | 0 (0.00) | |
| 5 | 0 (0.00) | 0 (0.00) | |
| 2nd | 6.766 | ||
| 0 | 0 (0.00) | 2 (5.00) | |
| 0.5 | 4 (5.88) | 4 (10.00) | |
| 1 | 41 (60.29) | 18 (45.00) | |
| 2 | 18 (26.47) | 13 (32.50) | |
| 3 | 5 (7.35) | 2 (5.00) | |
| 4 | 0 (0.00) | 0 (0.00) | |
| 5 | 0 (0.00) | 1 (2.50) | |
| 3rd | 16.187† | ||
| 0 | 0 (0.00) | 0 (0.00) | |
| 0.5 | 6 (8.82) | 1 (2.50) | |
| 1 | 39 (57.35) | 10 (25.00) | |
| 2 | 19 (27.94) | 21 (52.50) | |
| 3 | 4 (5.88) | 7 (17.50) | |
| 4 | 0 (0.00) | 0 (0.00) | |
| 5 | 0 (0.00) | 1 (2.50) | |
| CDR-SOB | 22.362 | ||
| 1st | |||
| 1.0 | 2 (3.0) | 0 (0.0) | |
| 1.5 | 0 (0.0) | 1 (2.7) | |
| 2.0 | 2 (3.0) | 1 (2.7) | |
| 2.5 | 0 (0.0) | 2 (5.4) | |
| 3.0 | 0 (0.0) | 2 (5.4) | |
| 4.0 | 1 (1.5) | 0 (0.0) | |
| 4.5 | 7 (10.6) | 2 (5.4) | |
| 5.0 | 17 (25.8) | 7 (18.9) | |
| 5.5 | 4 (6.1) | 4 (10.8) | |
| 6.0 | 4 (6.1) | 3 (8.1) | |
| 6.5 | 1 (1.5) | 2 (5.4) | |
| 7.0 | 1 (1.5) | 1 (2.7) | |
| 7.5 | 1 (1.5) | 0 (0.0) | |
| 8.0 | 1 (1.5) | 1 (2.7) | |
| 8.5 | 1 (1.5) | 0 (0.0) | |
| 10.0 | 6 (9.1) | 3 (8.1) | |
| 10.5 | 1 (1.5) | 0 (0.0) | |
| 11.0 | 5 (7.6) | 1 (2.7) | |
| 12.0 | 1 (1.5) | 3 (8.1) | |
| 13.0 | 3 (4.5) | 1 (2.7) | |
| 14.0 | 2 (3.0) | 0 (0.0) | |
| 16.0 | 2 (3.0) | 0 (0.0) | |
| 17.0 | 2 (3.0) | 1 (2.7) | |
| 18.0 | 1 (1.5) | 1 (2.7) | |
| 20.0 | 1 (1.5) | 1 (2.7) | |
| 2nd | 34.451 | ||
| 1.5 | 2 (2.9) | 0 (0.0) | |
| 2.0 | 2 (2.9) | 2 (5.1) | |
| 3.0 | 0 (0.0) | 2 (5.1) | |
| 4.0 | 3 (4.4) | 1 (2.6) | |
| 4.5 | 4 (5.9) | 4 (10.3) | |
| 5.0 | 16 (23.5) | 3 (7.7) | |
| 5.5 | 3 (4.4) | 4 (10.3) | |
| 6.0 | 8 (11.8) | 2 (5.1) | |
| 6.5 | 1 (1.5) | 0 (0.0) | |
| 7.0 | 3 (4.4) | 0 (0.0) | |
| 8.0 | 4 (5.9) | 3 (7.7) | |
| 8.5 | 0 (0.0) | 1 (2.6) | |
| 9.0 | 2 (2.9) | 3 (7.7) | |
| 9.5 | 0 (0.0) | 3 (7.7) | |
| 10.0 | 7 (10.3) | 3 (7.7) | |
| 11.0 | 0 (0.0) | 2 (5.1) | |
| 12.0 | 3 (4.4) | 2 (5.1) | |
| 13.0 | 1 (1.5) | 1 (2.6) | |
| 14.0 | 4 (5.9) | 0 (0.0) | |
| 16.0 | 1 (1.5) | 1 (2.6) | |
| 17.0 | 0 (0.0) | 1 (2.6) | |
| 18.0 | 1 (1.5) | 0 (0.0) | |
| 20.0 | 3 (4.4) | 0 (0.0) | |
| 28.0 | 0 (0.0) | 1 (2.6) | |
| 3rd | 39.406* | ||
| 1.5 | 2 (2.9) | 0 (0.0) | |
| 2.0 | 3 (4.4) | 1 (2.5) | |
| 2.5 | 1 (1.5) | 0 (0.0) | |
| 4.0 | 3 (4.4) | 0 (0.0) | |
| 4.5 | 3 (4.4) | 0 (0.0) | |
| 5.0 | 15 (22.1) | 1 (2.5) | |
| 5.5 | 9 (13.2) | 6 (15.0) | |
| 6.0 | 6 (8.8) | 2 (5.0) | |
| 6.5 | 1 (1.5) | 0 (0.0) | |
| 7.0 | 1 (1.5) | 1 (2.5) | |
| 8.0 | 1 (1.5) | 0 (0.0) | |
| 9.0 | 1 (1.5) | 0 (0.0) | |
| 10.0 | 5 (7.4) | 10 (25.0) | |
| 11.0 | 2 (2.9) | 6 (15.0) | |
| 12.0 | 5 (7.4) | 3 (7.5) | |
| 13.0 | 3 (4.4) | 1 (2.5) | |
| 14.0 | 3 (4.4) | 1 (2.5) | |
| 16.0 | 0 (0.0) | 3 (7.5) | |
| 17.0 | 1 (1.5) | 1 (2.5) | |
| 18.0 | 0 (0.0) | 3 (7.5) | |
| 19.0 | 1 (1.5) | 0 (0.0) | |
| 20.0 | 2 (2.9) | 0 (0.0) | |
| 28.0 | 0 (0.0) | 1 (2.5) |
Table 4.
| Maintained/improved (N=68) | Deteriorated (N=40) | p (group) | p (time) | p (group×time) | |
|---|---|---|---|---|---|
| 1st | 1.44±0.69 | 1.19±0.75 | 0.317 | <0.001 | <0.001 |
| 2nd | 1.38±0.66 | 1.43±0.92 | |||
| 3rd | 1.35±0.65 | 2.00±0.85 |
Table 5.
| Maintained/improved (N=68) | Deteriorated (N=40) | Independent t-test | |
|---|---|---|---|
| 1st | 1.44±0.69 | 1.19±0.75 | 1.790 |
| 2nd | 1.38±0.66 | 1.43±0.92 | -0.280 |
| 3rd | 1.35±0.65 | 2.00±0.85 | -4.200† |
| Paired t-test | |||
| 1st-2nd | 2.046* | -1.439 | |
| 2nd-3rd | 1.654 | -5.359† |
Table 6.
| Variable | B | Standard error | Wald | HR | 95% CI |
|---|---|---|---|---|---|
| Recall | -0.393 | 0.137 | 8.171 | 0.675† | 0.516-0.884 |
| Changes of activity in COVID-19 | -0.454 | 0.181 | 6.295 | 0.635* | 0.445-0.905 |
REFERENCES







