Structural and Functional Neural Alterations in Internet Addiction: A Study Protocol for Systematic Review and Meta-Analysis
Article information
Abstract
A growing number of neuroimaging studies have revealed abnormal brain structural and functional alterations in subjects with internet addiction (IA), however, with conflicting conclusions. We plan to conduct a systematic review and meta-analysis on the studies of voxelbased morphometry (VBM) and resting-state functional connectivity (rsFC), to reach a consolidated conclusion and point out the future direction in this field. A comprehensive search of rsFC and VBM studies of IA will be conducted in the PubMed, Cochrane Library, and Web of Science databases to retrieve studies published from the inception dates to August 2021. If the extracted data are feasible, activation likelihood estimation and seed-based d mapping methods will be used to meta-analyze the brain structural and functional changes in IA patients. This study will hopefully reach a consolidated conclusion on the impact of IA on human brain or point out the future direction in this field.
INTRODUCTION
With the widespread use of the internet around the world, the way of communication has changed tremendously. By the end of January 2022, there are over 5.2 billion active internet users, accounting for 66.2% of the global population (http://www.internetworldstats.com/stats.htm). The internet has become an indispensable role in modern life, however, while it has brought unprecedented benefits, internet addiction (IA) has also become a major problem plaguing the public.
IA refers to internet users’ obsession with the internet due to their long-term use, involving smartphone addiction, social media addiction, and internet gaming addiction (IGD) [1]. These phenomena will eventually lead to various negative effects, such as craving [2,3], impulsiveness [4], response inhibition [5], emotional disturbance [6], reduced executive function [7], and even cognitive disorders [8,9]. Besides, it also exposes people to sleep disorders [10], attention deficit hyperactivity disorder, depression, and anxiety [11-13]. Ultimately, concerns about IA increase over time.
Currently, magnetic resonance imaging (MRI), as a noninvasive neuroimaging tool, has been widely used to study brain characteristics. As the attention on IA increased, related neuroimaging studies have also emerged, especially voxelbased morphometry (VBM) studies and resting-state functional connectivity (rsFC) studies. VBM can reflect the gray matter (GM) structure of the brain, such as the significantly reduced volume of GM in the anterior cingulate cortex (ACC) [4], dorsolateral prefrontal cortex (DLPFC) [14], and supplemental motor area (SMA) [15] of patients with IA. rsFC is effective in detecting cerebral large-scale network dysfunction. Studies in this domain demonstrated that the rsFC was increased in insula [16], posterior cingulate cortex (PCC) [17], and precuneus [18] of IA patients, while the rsFC was decreased in SMA [18], inferior parietal lobule [19], and superior frontal gyrus (SFG) [20]. These studies suggest that IA not only affect the overall function of the brain but also lead to abnormalities in brain structure. However, not all results across these studies are consistent, therefore, a meta-analysis is needed to synthesize all current research to find a consistent conclusion.
At present, activation likelihood estimation (ALE) and seed-based mapping (SDM) are the two most used methods in the meta-analysis of neuroimaging [21,22]. ALE is easy for operation and strict for multiple comparison correction. However, it cannot synthesize both negative and positive outcomes. SDM can synthesize both positive and negative results, providing multimodal analysis, but the correction is not as strict as ALE. Considering both methods have advantages and disadvantages, conclusions obtained by using only one method may be biased.
Therefore, this protocol aims to comprehensively explore structural and functional brain alterations in individuals with IA by combining ALE and SDM. This study was registered in PROSPERO (CRD42021277662).
METHODS
The meta-analysis that integrates the latest neuroimaging studies will be conducted through ALE and SDM to synthesize the brain functional and structural changes associated with IA to better understand the effects of IA on the human brain, enabling targeted early intervention and treatment.
Criteria of selection for study
Criteria for inclusion
1) Diagnoses of IA in each study were based on Diagnostic and Statistical Manual of Mental Disorders-fifth edition, other quantitative assessment tools or both.
2) VBM studies or seed-based rsFC studies.
3) IA patients were compared with healthy controls (HCs).
4) Results were reported as coordinates in Montreal Neurological Institute (MNI) or Talairach space.
Criteria for exclusion
1) Not VBM study.
2) Not seed-based rsFC approach.
3) Studies only reported region of interest findings.
4) Longitudinal or intervention studies that did not compare with baseline state.
5) No HCs.
Search methods
Electronic searches
From the inception dates to August 2021, the following databases will be searched: PubMed, the Cochrane Library, and Web of Science. The searching strategy of the PubMed database is presented in Table 1.
Searching other resources
Additional studies will be further identified based on a list of all identified publications, including previous reviews and meta-analyses, as well as a reference list of the selected studies.
Data collection
Selection of studies
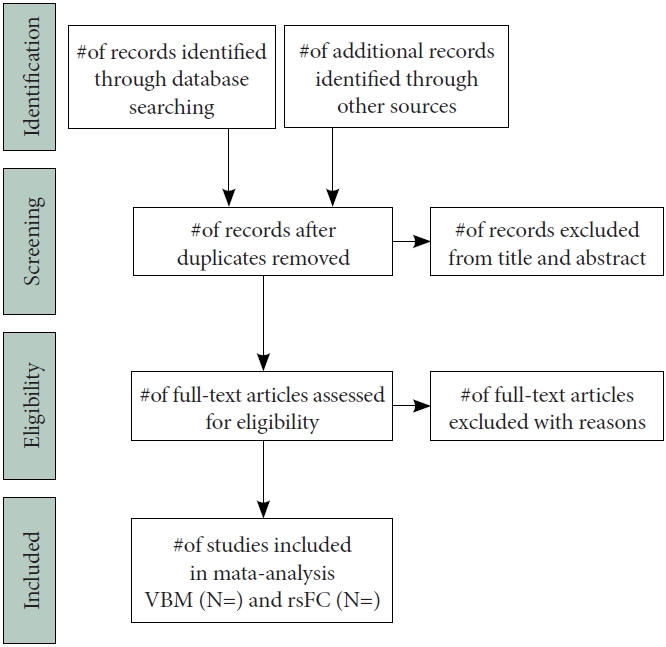
The study selection process will be consulted before publication selection. After an electronic search, the results will be output to a database created with Endnote software. Publications obtained from other sources will also be entered into the same database. Two authors will screen the included articles independently, any differences will be settled in consultation with a third researcher. First, the investigators will screen studies in the title/abstract, and then within the full text to determine whether to include them. The selection diagram for the study is shown in Figure 1. All reasons for exclusion will be listed in this report.
Data extraction
Information about the identified studies will be extracted, including the following data: first author name; published year; sample size for each group; comorbidity; mean age; diagnostic criteria; machine information; application of multiple comparison correction and coordinates of abnormal brain regions.
Quality assessment
Currently, there is no consensus on the quality assessment criteria for neuroimaging studies. Previous reviews of neuroimaging systems usually set quality assessment tools based on their studies. For this meta-analysis, we will use the Newcastle-Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm) as the basis for quality assessment and checked systematically for data acquisition, experimental design, diagnostic criteria, multiple comparison correction.
Data analysis
ALE meta-analysis
Brain regions with structural abnormalities and brain regions with functional abnormalities will be analyzed separately using the ALE technique, which is a voxel-based method for finding convergence across neuroimaging experiment coordinates [23,24]. First, the coordinates reported in Talairach space will be transformed into the MNI space using the “convert foci” option implemented in the Ginger ALE toolbox [25-27]. Then, the coordinates of MNI space will be input into ALE software. Based on recommendations [28], ALE maps will be set with a threshold at p<0.05 using a cluster level family-wise error (FWE) with a cluster forming threshold at voxel level p<0.001. The p-values in our analysis will be generated from 5,000 permutations.
SDM meta-analysis
SDM technology emerged after the ALE method. It is a common method for coordinate-based meta-analysis. Anisotropic effect size seed-based d mapping (AES-SDM) software has been used in many meta-analyses in the past [29,30]. To improve the reliability of the results and to compare the consistency of the SDM and ALE methods, we will investigate the VBM study and the rsFC study separately by using not only the latest version of the ALE software but also the latest version of the SDM software: seed-based d mapping with permutation of subject images (SDM-PSI). First, the peak coordinates of significant clusters and the related t-values will be extracted. If the original study reported z-scores or p-values, we will convert them to t-values using the online tools available on the SDM website [31]. Second, as a pre-processing step, the lower and upper effect-size bounds will be estimated. SDM-PSI calculates an image of the lowest potential effect size of each voxel and an image of the largest potential effect size of each voxel. A 20 mm full-width half maximum (FWHM) anisotropic Gaussian kernel and 2 mm voxel size will be used for preprocessing [31]. Third, the maximum likelihood estimation (MLE) of the most likely effect size and its standard error will be performed. The MLE will be performed according to standard SDM-PSI parameters [31]. Fourth, FWE correction based on threshold-free cluster enhancement will be used to thresholding the significant results [31]. The corrected p-value threshold will be 0.05, and the minimum cluster will be 10 voxels.
Heterogeneity, sensitivity, and published bias
Between-study heterogeneity was examined to find the heterogeneous brain regions with Q statistics. To verify the stability and reliability of the study results, we performed a jackknife sensitivity analysis, discarding each dataset in turn and repeating the combined analysis with other datasets. To test the possible publication bias, we will establish a funnel plot based on SDM-PSI software, which calculates effect sizes through Hedge’s g [32]. If there is no obvious publication bias, the scatter plot will resemble a symmetrical inverted funnel [33].
Subgroup analyses or meta-regression
If sufficient studies are included, we will examine potential confounding factors using subgroup analyses or meta-regression. For example, individuals were divided into IGD, smartphone addiction, social media addiction, etc.
DISCUSSION
Although IA has not been recognized by the World Health Organization, there is a rising trend of IA among the younger generation, and the prevalence of IA among adolescents and young adults is alarming. The results of the current systematic review show that IA as a complex problem is closely related to age, gender, region, and other variables (e.g., increased individualism, decreased sociability, and enculturation) [34,35]. IA deserves wider attention in more fields. IA is a behavioral addiction, similar to substance addiction, mainly related to abnormal reward system [36,37]. Previous research has shown that addiction leads to disruption or rewiring of neural circuits in the reward system [38]. This process mainly involves dopamine (DA) pathways in the mesolimbic region. Dopaminergic neurons are located in the ventral tegmental area and project to the nucleus accumbens (NAs) [39]. Certain addictive behaviors, including IA, increase DA in NAs, therefore the development of IA is associated with dopaminergic involvement [40]. The purpose of this study is to design an objective and accurate method for systematic review and meta-analysis to investigate the structural and functional abnormalities of the brain in patients with IA.
IA, as neurological dysfunction, has been explored in a large number of neuroimaging studies. The resting state is a functional brain imaging method used to assess interactions between brain regions when subjects are not performing specific tasks. Existing resting-state MRI studies show that brain abnormalities in IA patients are mainly concentrated in the default mode network (DMN), executive control network, and salience network compared to HCs. Studies of IGD have shown a significant decrease in the amplitude of low frequency fluctuations (ALFF) in the left SFG and posterior cerebellar lobe, while the ALFF in the superior temporal gyrus was increased [41,42]. In patients with smartphone addiction, the ALFF in the ACC, indicates reduced activity in the ACC [43]. This may be a neural correlate of impaired self-awareness and emotion regulation in IA patients. IGD also showed low rsFC between the left SFG and the PCC, the right angular gyrus, and the right DLPFC [41]. In addition, IGD participants showed decreased rsFC between the DLPFC and the caudate nucleus, left dorsal putamen, and left medial prefrontal cortex, and increased rsFC between the PCC and DLPFC [18,44,45]. These brain regions are associated with executive function and decision-making. These alterations may be potential biomarkers reflecting inhibitory function in patients with IA. Meanwhile, previous studies demonstrated that IA subjects revealed increased local functional connectivity density (lFCD) values in the right DLPFC, left parahippocampal gyrus, and cerebellum, and the bilateral middle cingulate cortex and superior temporal pole, as well as decreased lFCD values in the right inferior parietal lobe, and bilateral calcarine and lingual gyrus [46]. These regions involve cognitive control networks, DMN, and visual attention networks, which may be potentially linked to lack of attention and uncontrolled network use in individuals with IA [46]. In further, it has been indicated that using resting-state features can classify IA and HC, reveal brain alterations after intervention, and even efficacy assessment, for example, using functional connectivity density (FCD) can successfully distinguish IA from HC with an accuracy of 82.5%, while using the FCD change ratio can successfully assess the efficacy of cognitive behavior therapy with a correlation efficient of 0.59 [47]. And related studies have used rsFC to display IA brain changes in patients before and after acupuncture treatment [48]. MRI studies also revealed structural disorders in the prefrontal striatum circuit in IGD patients [49]. Subjects with IGD have reduced gray matter volume in brain regions associated with executive control, such as the ACC and SMAs [4,15], similar to drug addiction [50]. Besides, task-state functional magnetic resonance imaging studies can be used to estimate which areas of the brain are activated by the stimuli associated with IA. A study showed greater activation in the cortical-limbic system during cue-inducing tasks, with the left orbitofrontal cortex and ventral striatum associated with cue responses in computer game players [51]. Compared to controls, participants with online game addiction made more errors in the Go/No Go task and showed reduced activation in the dorsolateral prefrontal and superior parietal lobes, suggesting that IGD leads to impaired response inhibition [5]. In a reward-related task, internet addiction disorder patients also showed increased activation in the orbitofrontal cortex on gain trials and decreased activation in the ACC on loss trials, implying enhanced reward sensitivity and reduced loss sensitivity [52]. Positron emission computed tomography studies found that patients with IGD showed low metabolism in the ACC, temporal lobe, frontal lobe, parietal lobe, and striatum, and low metabolic connectivity between temporal and limbic regions and motor and occipital regions [53]. Patients with IA had reduced levels of striatal dopamine receptors compared to controls [54]. These findings above are consistent with mechanisms of addiction, but specific neurobiological markers are uncertain and more research on IA is urgently needed.
However, inconsistencies remain and reliable conclusions cannot be drawn. For example, in a study of IGD, the rsFC of the posterior insula and SMA was weakened compared to HC [20]. But an opposite situation was found in another IGD study that the rsFC of posterior insula and SMA was increased [55]. These inconsistencies are partly due to differences in research methods and limited sample sizes. Therefore, meta-analysis is required to synthesize these research results to draw the most credible conclusions.
Notes
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Rui Liu, Bo Hu. Data curation: Hui-Lin Hu. Formal analysis: Jing-Ting Sun. Funding acquisition: Rui Liu. Investigation: Jun-Li Liu, Jing-Ting Sun. Methodology: Jun-Li Liu, Hui-Lin Hu, Hao-Yuan Wang. Project administration: Hao-Yuan Wang. Resources: Jun-Li Liu. Software: Yun-Xi Kang, Tian-Qi Chen. Supervision: Zhu-Hong Chen, Yu-Xuan Shang. Validation: Yun-Xi Kang, Tian-Qi Chen. Visualization: Yu-Ting Li. Writing—original draft: Jun-Li Liu, Jing-Ting Sun. Writing—review & editing: Rui Liu, Bo Hu.
Funding Statement
This work was supported by the Major Theoretical and Practical Issues in Philosophy and Social Sciences of Shaanxi Province research Project (2021ND0187 to RL) and 2020 Project of the 13th Five-Year Plan of Education Science of Shaanxi Province (SGH20Y1098 to RL).
Acknowledgements
The authors would like to thank Drs. Wu-Xun Cui, Si-Jie Xiu, from the Department of Radiology of Tangdu Hospital for their outstanding technique support. Our gratitude also goes for Profs. Jin-Lian Li, Liang-Wei Chen, and Jun-Ling Zhu from the Department of Radiology of Tangdu Hospital for helpful comments on the manuscript.