Changes in Functional Connectivity Between Default Mode Network and Attention Network in Response to Changes in Aerobic Exercise Intensity
Article information
Abstract
Objective
Aerobic exercise may be associated with changes in brain activity within the default mode network (DMN) and dorsal attention network (DAN). We hypothesized that changes in functional connectivity (FC) within the DMN and DAN might be most effectively activated by moderate-intensity exercise.
Methods
Resting-state functional magnetic resonance imaging scans and visuospatial attention tests after resting were performed before and after each of moderate- and high-intensity aerobic exercises (10 min each) in 15 healthy male volunteers.
Results
The reaction time during the attention test increased significantly, and the rate of correct responses decreased from moderate-intensity exercise condition to high-intensity exercise condition. FC within the DMN under high-intensity exercise condition was higher than that under pre-exercise and moderate-intensity exercise conditions. FC within the DAN under moderate-intensity exercise condition was the highest, whereas FC between the DMN and DAN under moderate-intensity exercise condition was the lowest. Changes in cognitive domain functions were associated with changes in FC between the DMN and DAN.
Conclusion
Our results support the inverted-U hypothesis of maximum arousal efficacy during moderate exercise. Both cognitive domains, namely, the attention system and brain activity domains, may be better under moderate-intensity exercise than under high-intensity exercise.
INTRODUCTION
Aerobic exercise and O2 saturation in the brain
Aerobic exercise can enhance neurocognitive functions, including attention, executive function, memory, and working memory [1,2]. The acute effects of aerobic exercise may be associated with an increase in cognitive task-related arousal [3] and enhancement of cognitive performance efficacy [4]. Yanagisawa et al. [5] reported that one short bout of moderate aerobic exercise can lead to an increase in brain activation within the dorsolateral prefrontal cortex in response to a Stroop test.
Several studies have quantified the effects of aerobic exercise on cognitive functions and brain activity [6-8]. The invertedU hypothesis states that the optimal performance of cognitive functions can be achieved at an intermediate arousal level; however, the maximum performance of this function cannot be reached at low and high arousal levels [9]. In accordance with the inverted-U hypothesis, several studies have reported the best improvements in cognitive function in response to moderate-intensity exercise [5,7]. However, different cognitive function tests and various exercises have been used, and consistent results have not been obtained [6-8]. Moreover, few studies have reported changes in brain activation in response to different aerobic exercise levels.
Attention, exercise, and O2 consumption in the brain
Visuospatial attention capacity is strongly associated with individual differences in real-world tasks [10] and sports performance [11]. Guo et al. [12] suggested that sports training can enable athletes to develop efficient neural networks that engage in visuospatial tasks. Aerobic exercise decreases resting-state cerebral blood flow and increases cerebrovascular reactivity in response to performance tasks [13].
In our previous study on children with attention deficit hyperactivity disorder, aerobic exercise increases brain activity within the frontal and temporal cortices, which are associated with the attention system [14].
Breathing, O2 saturation, and brain network
Among several brain networks, the default mode network (DMN) is involved in breathing, heart rate, and respiration, which are related to aerobic exercise [15-17]. In addition, brain activation between the DMN and dorsal attention network (DAN) is affected by aerobic exercise [18-21].
The DMN is constituted by areas deactivated during outside stimulation or goal-oriented tasks but not at rest [15]. Anatomically, it is composed of the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), precuneus, and angular gyrus. The breath-by-breath O2–CO2 exchange ratio is associated with functional connectivity (FC) among the DMN components, including the precuneus and PCC [16]. The left and right anterior medial frontal areas are sensitively associated with heart rate and respiration [17]. The DMN is activated in response to thinking [18], and its activity is negatively correlated with that of attention networks [19].
The DAN is associated with the voluntary orientation of visuospatial attention [20]. It is referred to as a task-positive network because it is activated during attention-demanding tasks [21]. Anatomically, it is composed of the intraparietal sulcus (IPS) and frontal eye field [20]. It is also associated with hypercapnia (CO2 ventilation) [22].
Hypothesis
Aerobic exercise can enhance cerebral blood flow, which changes the brain FC within the DMN and DAN. On the basis of the inverted U-hypothesis, we hypothesized that changes in brain activity within the DMN and DAN might exhibit the most effective activation at moderate exercise intensity.
METHODS
Participants
Fifteen healthy male participants were recruited via advertisements posted at the Chung-Ang University advertisement internet board. Before advertisements, the protocol of resting-state functional magnetic resonance imaging (rs-fMRI) scanning and two-time aerobic exercise was discussed by two professors with a major in brain science, two professors with a major in sports science, and five students with a major in sports education. The inclusion criteria were as follows: 1) male sex, 2) age of 20–25 years, and 3) absence of psychiatric disease or medical illness, including respiratory or orthopedic diseases. The exclusion criteria were as follows: 1) history of head trauma with loss of consciousness, seizure disorder, brain tumor, or cerebrovascular accident; 2) history of substance abuse or claustrophobia; and 3) intelligence quotient (IQ) of <80. The research protocol was approved by the Institutional Review Board of Chung-Ang University (1041078-202108-HR-261-01), and written informed consent was obtained from all the participants.
Study procedure
The following steps were performed: pre-exercise computerized mental rotation test (visuospatial attention test; 5 min), pre-exercise rs-fMRI, moderate-intensity exercise (10 min), rest (10 min), computerized mental rotation test (visuospatial attention test; 5 min), and rs-fMRI after moderate-intensity exercise; and high-intensity exercise (10 min), rest (10 min), computerized mental rotation test (5 min), and rs-fMRI after high-intensity exercise (Figure 1). These two conditions were performed on same day.
A 15-min period was allocated for resting+mental rotation test between exercise and rs-fMRI [23]. The heart rate was assessed using a multi-sensor activity tracer (Fitbit AltaHR; Fitbit Inc., San Francisco, CA, USA) on the nondominant wrist during exercise. The belt speed of treadmill walking was adjusted for a warm-up period (5 min) to prepare for the exercise session and enrich the target heart rate for each exercise intensity.
Exercise intensity was determined according to the percentage of the maximal volume of oxygen uptake (VO2max) and cardiorespiratory function (the total amount of oxygen that an individual can utilize) [24]. On the basis of other studies on exercise intensity, moderate and high intensities were determined at 65% VO2max [25] and 80% VO2max, respectively [25]. The target heart rate (THR) was calculated using the Karvonen equation: THR = (HRmax–HRrest) × %intensity desired + HRrest [26]. HRmax was calculated using the “220 age” formula [27]. Considering 2–8 minutes of non cortical hemodynamic variables, including skin blood flow, middle cerebral artery flow, and time for the heart rate to return to baseline [28], two rest periods of 10 min were set before the rs-fMRI scan.
The computerized mental rotation test was run on a window-based table. A pair of 3D objects was presented on a computer screen in various ways: same shapes and rotations, same shapes but different rotations, different shapes but same rotations, different shapes, and different rotations. They were then rotated on a certain axis to a certain degree (0°, 60°, 90°, 120°, or 180°). In response to the presented type of the two 3D objects, the participants were asked to determine whether the two 3D objects were the same or different. During the test, which has a good test-retest reliability [29], the number of correct responses and the mean reaction time from the presentation of 3D objects to determination were recorded. The faster the reaction times and the higher the number of correct responses, the better the visuospatial attention.
The IQ of the participants was assessed using the Korean Wechsler Adult Intelligence Scale [30], which has an internal consistency of 0.78–0.94 [30].
Imaging processing and analysis
Resting-state brain activity data were obtained through fMRI performed using a 3.0 T scanner (Philips Achieva 3.0 T TX MRI scanner; repetition time = 3 s, 12 min scan, 240 volumes, 128 × 128 matrix, 40 slices at 4.0 mm slice thickness). Data were preprocessed and processed using the Data Processing Assistant for Resting-State fMRI (DPARSFA; http://restfmri.net/forum), a plug-in software that works with Statistical Parametric Mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the Resting-State fMRI Data Analysis Toolkit (REST; http://resting-fmri.sourceforge.net). During preprocessing, the following procedures were performed: despiking (AFNI: 3dDespike), motion correction (SPM 12b), co-registration to magnetization-prepared rapid acquisition gradient echo image (SPM 12b), normalization to Montreal Neurological Institute space, temporal detrending (Matlab: detrend.m), bandpass filtering (Matlab: idealfilter.m), and cerebrospinal fluid, white matter, and facial soft tissue degrading (Matlab), as described in previous studies [30]. For the correction of head movement, the voxel-wise regression of identically bandpass-filtered time series of six head motion parameters was applied to realignment steps with six rigid-body parameters characterizing the estimated subject motion of each subject. Global signal regression was not performed [31,32].
Images were corrected for slice acquisition time differences, realigned, normalized, and spatially smoothened with a 6 mm full-width half maximum kernel. Functional images were detrended and temporally bandpass filtered to 0.01–0.08 Hz to remove signal artifacts (e.g., heartbeat, respiration).
Eight regions in the two brain networks (four from the DMN: MPFC, right/left angular gyrus, PCC; four from the DAN: right/left frontal eye field, right/left inferior parietal sulcus [IPS]) were extracted from the AAL atlas of the brain (https://www.nitrc.org) [33]. Fisher-transformed correlation coefficients were measured for each pair of regions of interest (ROIs) in each subject. FC was calculated between ROIs by using the CONN-fMRI FC toolbox (ver.15; www. Nitrc.org/projects/conn). Between-group effects were considered significant at a cluster-level false discovery rate (FDR) with p<0.05.
Statistical analysis
Changes in the reaction time and rate of correct responses in the mental rotation test from baseline to post-moderate and post-high-intensity exercises were analyzed via repeated-measures analysis of variance (ANOVA). Changes in FC between ROIs from pre-exercise to moderate- and high-intensity exercise conditions were also examined using repeated-measures ANOVA. Correlations between changes in the rate of correct responses in the mental rotation test and changes in FC between ROIs were evaluated through Pearson correlations.
RESULTS
Demographic and visuospatial attention test characteristics
The mean age, height, weight, body mass index, and years of education of all participants were 23.2±1.6 years, 175.7±4.2 cm, 75.4±6.2 kg, 24.1±2.0 kg/m2, and 15.0±1.7 years, respectively (Table 1).
The mean rate of correct responses and reaction time in the visuospatial attention test were 71.5%±12.1% and 2.8± 1.1 s, respectively.
Changes in visuospatial attention in response to exercise intensity
The reaction time in the mental rotation test changed significantly in response to exercise intensity (F=19.27, p<0.01), but it did not change from pre-exercise to moderate-intensity exercise condition (z=0.91, p=0.36). However, from moderate-intensity exercise condition to high-intensity exercise condition, the reaction time increased significantly (z=3.41, p<0.01) (Figure 2A).
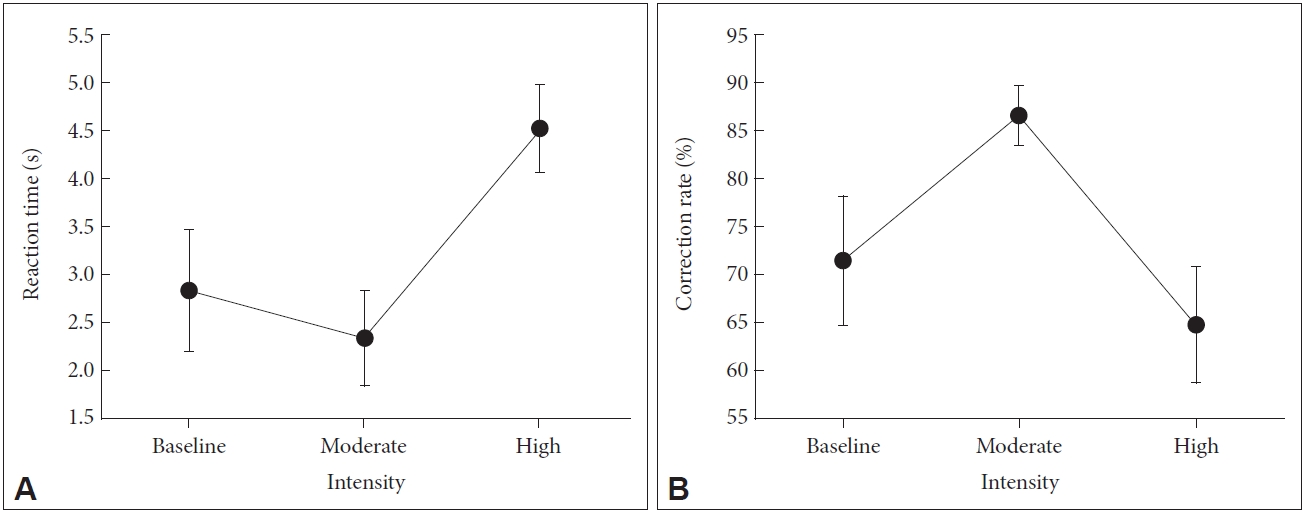
Changes in visuospatial working memory in response to exercise intensity. A: Changes in reaction time in the mental rotation test in response to exercise intensity, F=19.27, p<0.01. B: Changes in the rate of correct responses in the mental rotation test in response to exercise intensity, F=22.13, p<0.01.
The rate of correct responses in the mental rotation test changed significantly in response to exercise intensity (F=22.13, p<0.01). Specifically, it increased from pre-exercise to moderate-intensity exercise condition (z=2.50, p<0.01). However, from moderate-intensity exercise condition to high-intensity exercise condition, the rate of correct responses decreased (z=3.41, p<0.01) (Figure 2B).
Changes in brain FC in response to changes in exercise intensity
In the DMN, FC from the PCC to the MPFC (F=5.69, p<0.01) and from the PCC to the right angular gyrus (F=7.39, p<0.01) increased from pre-exercise to high-intensity exercise condition (Figure 3A and B).
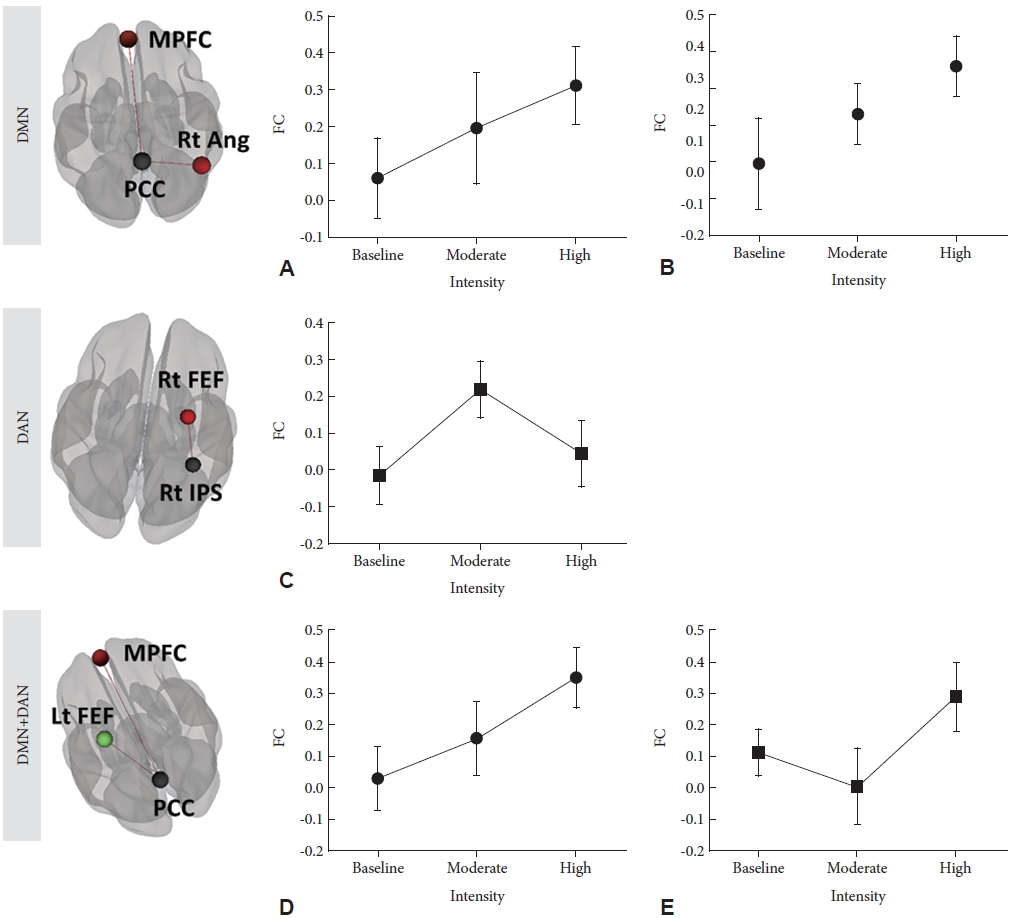
Changes in brain functional connectivity in response to changes in exercise intensity. Changes in functional connectivity (FC) (A) from the posterior cingulate cortex (PCC) to the medial prefrontal cortex (MPFC) (F=5.69, p<0.01); (B) from the PCC to the right angular gyrus (Rt Ang; F=7.39, p<0.01); (C) from the right inferior parietal sulcus to the right frontal eye field (F=9.31, p<0.01); (D) from the PCC to the MPFC (F=11.5, p<0.01); and (E) from the PCC to the left frontal eye field (F=11.4, p<0.01) in response to changes in exercise intensity. DMN, default mode network; DAN, dorsal attention network; Rt FEF, right frontal eye field; Rt IPS, right inferior parietal sulcus; Lt FEF, left frontal eye field.
In the DAN, FC from pre-exercise to high-intensity exercise condition changed significantly (F=9.31, p<0.01). From pre-exercise to moderate-intensity exercise condition, FC from the IPS to the right frontal eye field increased. However, from moderate-intensity exercise condition to high-intensity exercise condition, the beta FC from the IPS to the right frontal eye field decreased (Figure 3C).
With respect to both the DMN and DAN, FC from the PCC to the MPFC increased from pre-exercise to high-intensity exercise condition (F=11.5, p<0.01). FC from the PCC to the left frontal eye field decreased from pre-exercise to moderate-intensity exercise condition (z=3.01, p<0.01), but FC from the PCC to the left frontal eye field increased from moderate-intensity to high-intensity exercise condition (z=2.56, p=0.01) (Figure 3D and E).
Correlations between changes in brain FC and those in visuospatial attention
From pre-exercise to moderate-intensity exercise condition, changes in the rate of correct responses were correlated with changes in FC from the IPS to the right frontal eye field (r=0.57, p=0.027). However, their difference was not statistically significant.
From moderate-intensity exercise condition to high-intensity exercise condition, changes in the rate of correct responses were negatively correlated with changes in FC from the PCC to the MPFC (r=-0.816, p<0.0001). From moderate-intensity exercise condition to high-intensity exercise condition, changes in reaction time were correlated with changes in FC from the PCC to the MPFC (r=0.818, p<0.0001) (Figure 4).
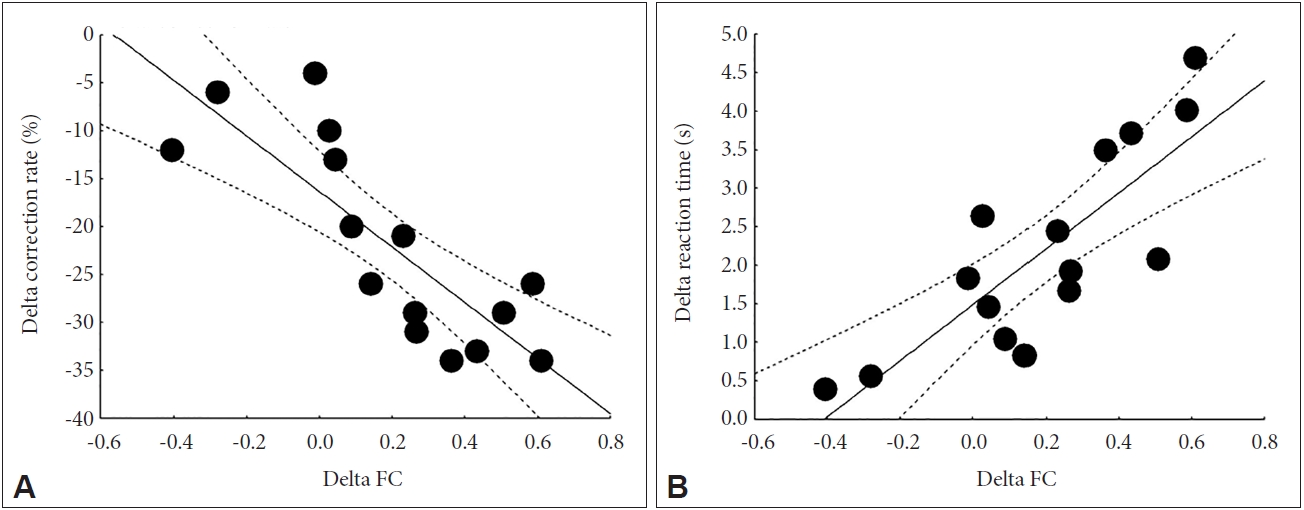
Correlations between changes in brain functional connectivity and changes in visuospatial attention. A: From moderate-intensity exercise condition to high-intensity exercise condition, the correlation coefficient between changes in the rate of correct responses and changes in functional connectivity (FC) from the posterior cingulate cortex to the medial prefrontal cortex was r=-0.816 (p<0.0001). B: From moderate- intensity exercise condition to high-intensity exercise condition, the correlation coefficient between changes in reaction time and changes in FC from the posterior cingulate cortex to the medial prefrontal cortex was r=0.818 (p<0.0001).
DISCUSSION
The current results support the inverted-U hypothesis of the maximum arousal efficacy during moderate exercise. Both cognitive domains, namely, the attention system and brain activity domains, might be better with moderate-intensity exercise than with high-intensity exercise. Moreover, changes in cognitive domain functions were associated with changes in FC between the DMN and DAN.
Changes in visuospatial attention in response to exercise intensity
The reaction time and rate of correct responses in the mental rotation test were significantly associated with exercise intensity in the current study. Reaction time was slow during high-intensity exercise, and the rate of correct responses was the highest during moderate-intensity exercise.
These results were consistent with previous findings [34,35]. Decroix et al. [34] reported that aerobic exercise significantly improves the speed of information processing (reaction time) in healthy participants. Lefferts et al. [35] demonstrated that the hypoxic condition induced by acute aerobic exercise can slow down the reaction time but cannot change the accuracy in N-back and flanker tasks.
Changes in brain FC in response to changes in exercise intensity
FC within the DMN during high-intensity exercise was higher than that at baseline and during moderate-intensity exercise. FCs within the DAN and between DMN and DAN during moderate-intensity exercise were the highest and lowest, respectively.
For maximum attentive function, the switching between the DMN and attention network (ATN) should be flexible [36]. The flexible FC between the DMN and ATN is likely a result of the decreased FC within the DMN, increased FC within the ATN, or both [36]. Interestingly, the modulation of respiration is possibly associated with the DMN [4]. In the current study, the rate of correct responses and reaction time of cognitive functions were negatively correlated with the FC within the DMN during high-intensity exercise. During high-intensity exercise, brain activity within the DMN may increase to modulate respiration, while brain activity within the ATN may decrease to compensate for respiration [4,35]. Changes in brain activity within the DMN and ATN could increase the FC between DMN and ATN, thereby decreasing the attentive function.
Many studies with variable assessment modules have suggested cerebral blood flow modulation in response to aerobic exercise intensity [37-40]. In duplex Doppler ultrasound studies, aerobic exercise can increase blood flow within the internal carotid and vertebral arteries until moderate exercise intensity is achieved. However, blood flow does not increase during high-intensity exercise [38,39]. Some studies have suggested that high-intensity aerobic exercise can lead to a decline in cerebral blood flow [40]. However, global cerebral blood flow is only 15% of the cardiac output [41], and changes in regional cerebral blood flow can be represented by heavy or high-intensity exercise [42]. Few studies have suggested a direct correlation between regional cerebral blood flow and changes in brain function. In addition, the cumulative effects of aerobic exercise on brain activity should be estimated in future studies.
Considering the disadvantages of regional cerebral blood flow studies, further studies on brain connectivity between networks may provide new insights into cognitive and brain activity changes in response to aerobic exercise.
Limitations
The current study has several limitations. First, the results obtained using a small all-male sample could not be generalized to the general population. Therefore, future studies with larger sample sizes are warranted. However, the current sample size could accurately reflect brain activity in response to aerobic exercise. Second, the participants in the current study were limited to young men. Future studies should assess the effects of aerobic exercise on the brain activity of individuals with a wide age range. Finally, although we monitored the heart rate and removed signal artifacts via image preprocessing techniques, respiration and heart rate could affect the results of brain activity.
In conclusions, cognitive accuracy and speed are lower during high-intensity exercise than during moderate-intensity exercise. During moderate-intensity exercise, the FC between the DMN and DAN was most efficiently activated.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Doug Hyun Han. Data curation: Young-woo Ko. Formal analysis: Sun Mi Kim. Funding acquisition: Doug Hyun Han. Investigation: Young-woo Ko. Methodology: Kyoung Doo Kang. Project administration: Doug Hyun Han. Resources: Doug Hyun Han. Software: Kyoung Doo Kang. Supervision: Doug Hyun Han. Validation: Sun Mi Kim. Visualization: Sun Mi Kim. Writing—original draft: Doug Hyun Han. Writing— review & editing: Doug Hyun Han.
Funding Statement
This work was supported by the Ministry of Culture, Sports, and Tourism and Korea Creative Content Agency (Project Number: R2020040186).


