The Effect of TNF-alpha rs1800629 Polymorphism on White Matter Structures and Memory Function in Patients With Schizophrenia: A Pilot Study
Article information
Abstract
Objective
This study investigated the effect of TNF-α rs1800629 polymorphism on white matter integrity and memory function in patients with schizophrenia.
Methods
Fifty-five participants with schizophrenia were enrolled in this study. They were genotyped for TNF-α rs1800629 polymorphism and underwent diffusion tensor imaging. Memory function was assessed using the Rey–Kim memory test. Participants with schizophrenia were grouped into GG homozygotes and A-allele carriers.
Results
Compared to GG homozygotes, A-allele carriers had significantly lower scores for immediate and delayed recall and recognition of verbal memory and showed significantly lower fractional anisotropy in extensive brain regions. Lower total scores in immediate and delayed recall of verbal memory, immediate recall of visual memory, and figure copy of visual memory were significantly correlated with decreased mean fractional anisotropy in the white matter tracts of the corresponding brain regions.
Conclusion
Our findings suggest that the A-allele, which is associated with higher levels of TNF-α expression, correlates with lower connectivity of the fronto-temporal white matter compared to that in GG homozygotes. Impaired fronto-temporal connectivity may be associated with genetic vulnerability to schizophrenia, leading to verbal and visual memory deficits in patients with schizophrenia.
INTRODUCTION
Schizophrenia is characterized by dysconnectivity between different brain regions, which is mediated by disrupted white matter (WM) integrity [1,2]. Although the exact etiology of disrupted WM integrity in schizophrenia remains unknown, several genomic, blood, and neuroimaging studies have suggested that dysregulation of the inflammatory system mediated by cytokines might be linked to the pathophysiology of schizophrenia and can lead to changes in mood, cognition, and behavior [3-6]. In particular, the polymorphisms of tumor necrosis factor α (TNF-α), interleukin (IL)-10, and IL-6 have been strongly linked to the pathogenesis of schizophrenia [6,7], and the peripheral levels of these cytokines are higher in patients with schizophrenia than in healthy controls [8,9].
Among these cytokines, TNF-α, a key pro-inflammatory cytokine that acts through the TNF receptor, is likely associated with the pathogenesis of schizophrenia, as many studies have reported that the peripheral and central levels of TNF-α are elevated in patients with schizophrenia [10-12]. In addition to its key role in compromising the complicated events involved in immunity and inflammation [13], TNF-α has novel roles in central nervous system development and functions, such as nerve cell growth, neuronal plasticity, differentiation, cognition, and behavior [14-16]. TNF-α signaling disruption has been linked to hippocampus structure abnormalities as well cognitive dysfunction [17,18]. Inflammatory pathways related to TNF-α may be involved in the pathogenesis of cognitive dysfunction in schizophrenia. Therefore, the neuroinflammatory effects of TNF-α have been attributed to cognitive dysfunctions. Patients with schizophrenia have been shown to have neurocognitive deficits and increased inflammatory cytokine levels; however, evidence supporting a link between these phenomena remains scarce. Although several single nucleotide polymorphisms (SNPs) within the TNF-α locus influence TNF-α production, in vitro studies show that the A allele of the -308G/A SNP increases TNF-α transcription [19,20]. A genetic disposition to produce increased levels of TNF-α, due to the presence of the -308A allele, may change the course of immune activity with an increased risk of disease [21].
Emerging evidence suggests that TNF-α may be associated with microstructural WM aberrations [22,23]. The serum levels of the pro-inflammatory cytokines, TNF-α, IFN-γ, and IL-8, were significantly and inversely associated with the fractional anisotropy (FA) in large overlapping networks of WM mostly located in the anterior region of the brain [22]. Additionally, the level of peripheral inflammatory markers, including TNF-α, IL-1β, and IL-10, inversely correlated with lower FAs in older adults with Alzheimer’s disease [24]. The association of inflammatory cytokines with WM integrity might be explained by the certain effects of cytokines on oligodendrocyte biology. As TNF receptors are expressed by oligodendrocyte progenitors, TNF receptors can impede the survival and differentiation of oligodendrocyte precursor cells by mediating reactive astrocytes to induce oligodendrocyte precursor cell apoptosis [25]. In response to insults, including chronic stress [26], activated microglia may provoke white and gray matter injury by producing pro-inflammatory cytokines, such as TNF-α [27], which may influence the cytotoxic effects on oligodendrocyte precursor cells, endothelial cells, and neurons [28].
To our knowledge, previous studies have not demonstrated an association between the TNF-α polymorphism, microstructural WM integrity, and severity of neurocognitive deficits in schizophrenia. Thus, the aim of this study was to explore the effect of the TNF-α rs1800629 -308G/A polymorphism on WM integrity in relation to neurocognitive impairment in patients with schizophrenia. We hypothesized that patients with schizophrenia who are A-allele carriers would have more disrupted WM integrity, as measured using diffusion tensor imaging (DTI)-derived FA, than those who are GG homozygotes. Second, we hypothesized that neurocognitive function in patients with schizophrenia would be associated with the TNF-α polymorphism and FAs in WM regions.
METHODS
Participants
Fifty-five patients with schizophrenia were recruited from the Department of Psychiatry at CHA Bundang Medical Center. All participants were between 18 and 65 years of age, of Korean descent, and were right-handed. All participants met the diagnostic criteria for schizophrenia from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) [29]. To only include patients with schizophrenia, the diagnostic interviews were conducted according to the Structured Clinical Interview for DSM-IV Axis I disorders by experienced psychiatrists. Patients were excluded if they presented with the following characteristics: 1) presence of Axis I or Axis II psychiatric disorders (based on DSMIV-TR criteria) other than schizophrenia, 2) a previous history of medical or neurological illness, 3) intellectual disability, or 4) contraindications for magnetic resonance imaging (MRI). Finally, fifty-five patients with schizophrenia who were prescribed antipsychotics including risperidone, paliperidone, olanzapine, clozapine, aripiprazole, and ziprasidone were included in this study.
All study procedures were evaluated and approved by the Institutional Review Board of CHA Bundang Medical Center (IRB no. 2020-02-015, 2021-03-001) in accordance with the latest version of the Declaration of Helsinki and principles of Good Clinical Practice. All participants provided written informed consent after a thorough explanation of the study procedure.
Clinical assessment
The clinical symptom severity of participants with schizophrenia was assessed using the Scale for the Assessment of Positive Symptoms (SAPS) [30] and the Scale for the Assessment of Negative Symptoms (SANS) [31] at baseline by experienced psychiatrists. The neurocognitive function of participants was assessed by clinically experienced psychologists using the Rey–Kim Memory Test (RKMT), a Korean standardized version of the Complex Figure Test (K-CFT), and Auditory Verbal Learning Test (K-AVLT) initially developed by Andre Rey [32]. The RKMT is known to evaluate defects in memory mechanisms. The K-AVLT comprises five successive presentations of a list of 15 words followed by immediate recall, a 20-minute delayed recall, and a 20-minute delayed recognition trial. Delayed recognition was assessed using a learning list of 15 words, from which participants were instructed to select words that they were presented from the worksheet. Raw scores for the delayed recognition trial, five acquisition trials, delayed recall trials, and three process composites were included in the statistical analysis. In the K-CFT, a complex figure was presented to the participants, and then they were asked to 1) copy the figure (copy) and 2) draw the figure immediately (immediate recall). After 20 minutes, delayed recall was examined. The performance was assessed as the sum of the scores for each of the 18 elements in the figure (maximum score=36).
Genotyping
Genotype data for the TNF-α rs1800629 -308G/A polymorphism were obtained from genome-wide genotyping data using the Affymetrix AxiomTM KORV1.1-96 Array. Participants with schizophrenia were genotyped using the AxiomTM 2.0 Reagent Kit (Affymetrix, Santa Clara, CA, USA) according to the manufacturer’s protocol. All the equipment and resources required for the AxiomTM 2.0 assay with automated target preparation are indicated in the AxiomTM 2.0 assay automated workflow user guide (P/N 702963). About 200 ng of genomic DNA was amplified and randomly fragmented into 25- to 125-base pair (bp) fragments. An additional fragmentation step reduced the amplified products into segments of approximately 25–50 bp in length, which were end-labeled using biotinylated nucleotides. The samples were denatured and transferred to a hyb tray and then prepared for hybridization in the GeneTitanTM Multi-Channel Instrument (Affymetrix). The hybridization step followed the GeneTitanTM Multi-Channel Instrument User’s Manual (P/N 08–0306) and used an AxiomTM Biobank Plus Genotyping Array KNIHv1.0. After ligation, the arrays were stained and imaged using the GeneTitanTM MC Instrument (Affymetrix). The images were analyzed using the AffymetrixTM GeneChipTM Command ConsoleTM Software User Manual (P/N 702569).
MRI data acquisition
For all participants, MRI data were acquired using a 3.0-T GE Signa HDxt scanner (GE Healthcare, Milwaukee, WI, USA) at CHA Bundang Medical Center. Diffusion-weighted images (DWI) were acquired using an echo planar imaging (EPI) sequence with the following parameters: repetition time, 17,000 ms; echo time, 108 ms; field of view, 240 mm; 144×144 matrix; slice thickness, 1.7 mm; and voxel size, 1.67× 1.67×1.7 mm3. A double-echo option was used to reduce the distortion effect of the eddy currents. An eight-channel coil and an array of spatial sensitivity encoding techniques (ASSET; GE Healthcare) with a sensitivity encoding (SENSE) factor of two were applied to reduce the effect of EPI spatial distortions. Seventy axial slices parallel to the anterior commissureposterior commissure line for the whole brain were acquired in fifty-one directions with a b-value of 900 s/mm2. Eight baseline scans at b=0 s/mm2 were also obtained. Using the leastsquares method, DTIs were extracted from DWIs (approximate scan time of 17 min).
Diffusion tensor imaging analysis
DTI data were analyzed using Tract-based Spatial Statistics (TBSS version 1.2) and Oxford Functional MRI of the Brain (FMRIB) Diffusion Toolbox in the FMRIB Software Library (FSL version 6.0; Oxford, UK, http://www.fmrib.ox.ac.jk/fsl/). DTI preprocessing was performed according to standard procedures using FSL. Image artifacts due to eddy current distortions were minimized by recording the images to the baseline scans. For the registered images, skull stripping was performed using the Brain Extraction Tool (BET). FA maps were created using the FMRIB diffusion toolbox. The FA data of participants were calculated by fitting a tensor model to the corrected diffusion data [33] and aligned to a common space (Montreal Neurologic Institute 152 standard) using a nonlinear image registration tool (FNIRT) to register the images to the standard FA template. The aligned FA maps were combined and applied to the original FA map to produce a standard-space version of the FA map. Next, a mean FA image was created by averaging the transformed FA images of all participants in standard space and narrowed to generate a mean FA skeleton representing only the center of all WM tracts common to the entire group. This was thresholded to FA >0.2 (TBSS default) to include only major WM fiber bundles.
Mean FA skeletons were multiplied using the Johns Hopkins University (JHU) DTI-based probabilistic tractography atlas. Next, WM regions that showed a significant difference in FAs between polymorphisms were extracted using threedimensional Slicer, version 3.6, to create a mask and conduct voxel-wise statistical analysis. Statistical analysis of DTI data was conducted through 5,000 permutations using permutation-based nonparametric inference within the framework of a general linear model. Threshold-free cluster enhancement (TFCE) was used to define clusters and adjust for multiple comparisons. In addition, an analysis of covariance with age, sex, intracranial volume (ICV), antipsychotic drug dose at scan, and duration of illness was performed to rule out the confounding effects of these variables on the results. The significance level of the data was set to TFCE-corrected p<0.01.
Statistical analyses
Clinical and sociodemographic characteristics and the neurocognitive function of GG homozygotes and A-allele carriers among participants with schizophrenia were compared using Mann–Whitney tests for continuous variables and chisquared tests for categorical variables. Partial correlation analyses after controlling for the covariates of variables, such as age and sex, were used to explore the relationships between mean WM FA values and memory function for exploratory analysis. Statistical analyses of non-imaging data were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics 25.0; IBM Corp.; Armonk, NY, USA). For all analyses, a two-sided p<0.05, was considered statistically significant.
RESULTS
Sociodemographic characteristics and neurocognitive function
The sociodemographic and clinical characteristics of the participants are summarized in Table 1. The A-allele frequency was 0.109. No statistically significant differences in sex, age, mean years of education, duration of illness, ICV, family history of schizophrenia spectrum disorders, total duration of antipsychotic treatment before the MRI scan, dosage of antipsychotic medication, SANS, or SAPS values were found between A-allele carriers and GG homozygotes. However, GG homozygotes had significantly higher CGI-S values than the A-allele carriers. Table 2 presents the mean RKMT scores, which are a measure of neurocognitive function, between GG homozygotes and A-allele carriers. Compared to GG homozygotes, A-allele carriers showed significantly poorer performance in verbal memory (immediate recall: U=159.0, p=0.043; delayed recall: U=152.5, p=0.031; delayed recognition: U=128.0, p=0.009).
Voxel-wise t-test comparison between GG homozygotes and A-allele carriers
In Figure 1, a voxel-wise t-test comparison revealed that the FA values were significantly lower across extensive brain regions, including the genu, body, and splenium of the corpus callosum; posterior limb and retrolenticular part of the right internal capsule; bilateral anterior, superior, and posterior corona radiata; right posterior thalamic radiation; right sagittal stratum; right external capsule; bilateral cingulate gyrus; right fornix and stria terminalis; bilateral superior longitudinal fasciculus; and right tapetum in A-allele carriers (median [interquartile range]=0.58 [0.55–0.61]) than in GG homozygotes (median [interquartile range]=0.63 [0.61–0.64]; U=44.0, p< 0.001) (Figure 2).

White matter regions that showed significantly lower FA values in A-allele carriers compared to GG homozygotes (TFCE-corrected p<0.01). FA, fractional anisotropy; TFCE, threshold-free cluster enhancement.
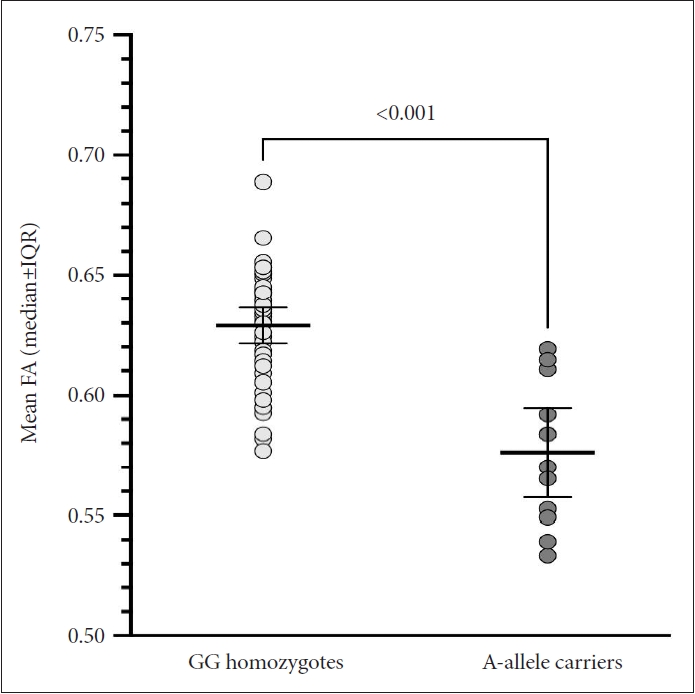
Scatter plot of mean FA extracted from the white matter regions showing statistical significance in the voxel-wise comparison between GG homozygotes and A-allele carriers. The mean FA values were significantly lower in A-allele carriers than in GG homozygotes (Mann–Whitney U=44.0, p<0.001). FA, fractional anisotropy; IQR, interquartile range.
Relationship between mean fractional anisotropy values of corresponding brain regions and memory function in participants with schizophrenia
In Figure 3, the correlational analysis showed that lower total scores in immediate recall of verbal memory, delayed recall of verbal memory, figure copy of visual memory, and immediate recall of visual memory were significantly correlated with the lower mean FAs of the corresponding brain regions between the two groups. Delayed recall of verbal memory, recognition of verbal memory, immediate recall of visual memory, and delayed recall of visual memory were not significantly correlated with the mean FAs of the corresponding brain regions.
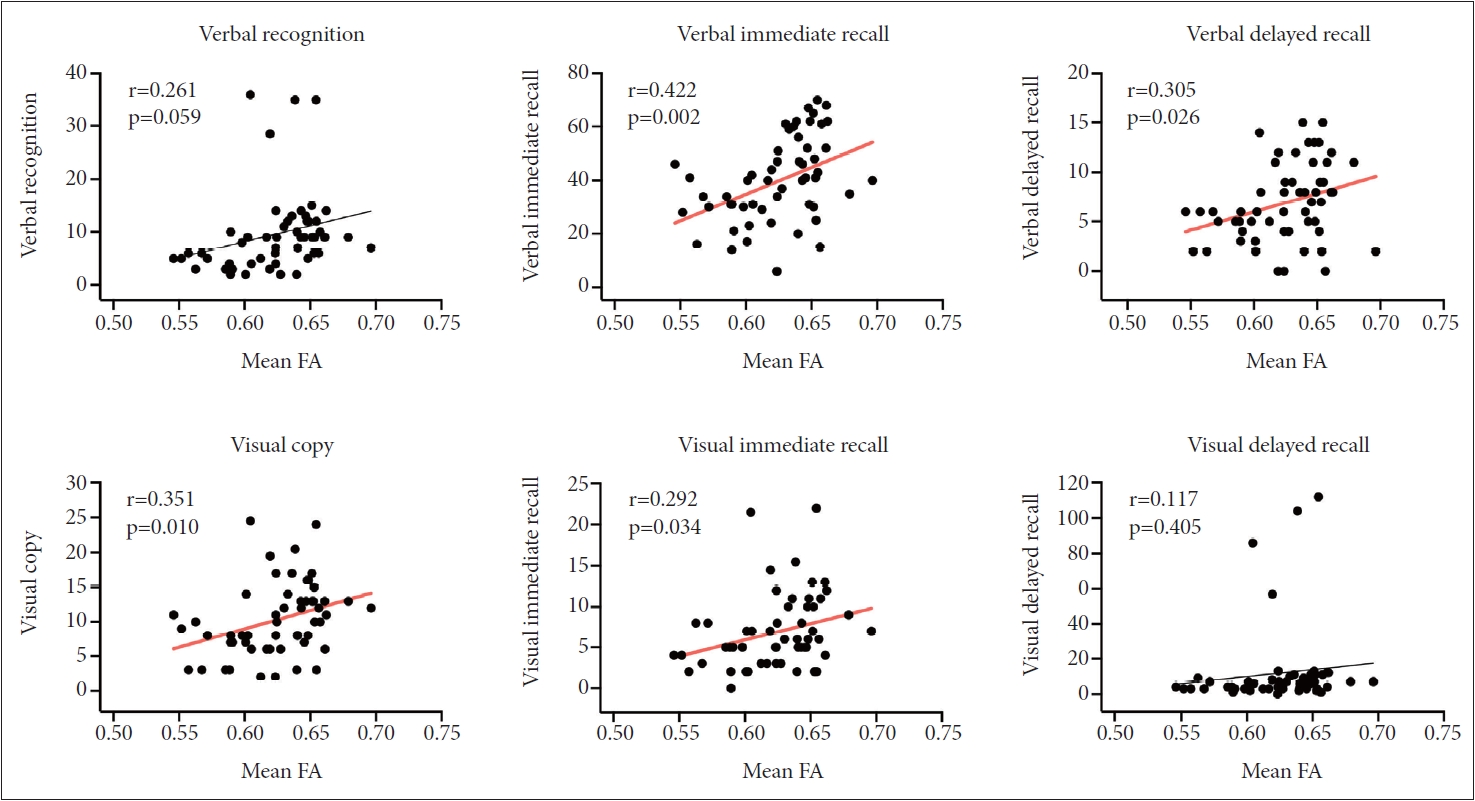
Correlation of memory function with mean FA values extracted from the significant cluster in the between-group voxel-wise comparison. Total scores in immediate recall of verbal memory, delayed recall of verbal memory, figure copy of visual memory, and immediate recall of visual memory were significantly correlated with the mean FA values. FA, fractional anisotropy.
DISCUSSION
The current study investigated the association between neurocognition, microstructural WM integrity, and the TNF-α rs1800629 -308G/A polymorphism in participants with schizophrenia. The findings demonstrated that A-allele carriers of the TNF-α rs1800629 -308G/A polymorphism in schizophrenia had significantly lower FAs in extensive brain regions and significantly impaired verbal immediate and delayed recall and verbal recognition memory function, as measured using the RKMT, than GG homozygotes. Moreover, we demonstrated a direct correlation between FAs in extensive corresponding brain regions and performance in verbal immediate and delayed recall, visual immediate recall, and visual copy memory function measured by the RKMT. Our findings suggest that the -308A allele in the TNF-α rs1800629 polymorphism in patients with schizophrenia may be attributed not only to more severe neurocognitive dysfunction but also to the dysconnectivity of extensive brain regions, especially fronto-temporal WM connectivity, leading to memory impairment. To our knowledge, this study is the first to provide evidence that polymorphisms in inflammatory cytokines may affect structural and functional dysconnectivity and neurocognitive impairment in patients with schizophrenia.
In the current study, the frequency of the A allele was 11%, verifying that the A allele tended to be less frequent than GG homozygosity documented in English (20%) [35], French (18%) [36], Danish (22%) [37], and Spanish subjects (9%) [38]. In this study, Aallele carriers had significantly impaired verbal memory function compared with GG homozygotes. Previous studies have shown that the A allele of the -308G/A polymorphism increases the expression of TNF-α in human B-cell lines [19]. A previous study suggested that the A allele, which has been associated with an increased risk of schizophrenia [39], is linked to higher peripheral TNF-α levels than GG homozygosity [40]. Patients with schizophrenia with higher serum TNF-α levels displayed more severe neurocognitive deficits, especially in the domains of visual learning, processing speed, reasoning, and problem-solving [41]. In studies by Maes et al. [42] and Al-Hakeim et al. [43], increased TNF-α levels were associated with episodic memory deficits and semantic and episodic memory deficits, respectively. However, neither study revealed any significant correlation between TNF-α levels and executive function, implying that increased TNF-α levels might be specifically associated with memory. Our findings extend previous findings that indicate a link between neurocognitive deficits and increased inflammation in patients with schizophrenia. Previous studies examining the association between TNF-α and neurocognition in patients with schizophrenia have reported inconsistent results, possibly due to the use of variable cognitive measures in previous studies [44-46]. Since previous studies did not examine memory function as a representative of neurocognition using the RKMT to compare GG homozygotes and A-allele carriers, we measured verbal and visual memory function using the RKMT and compared findings between GG homozygotes and A-allele carriers. As a result, this study suggests that the A-allele is associated with more severe impairment of verbal memory function, along with increased TNF-α expression.
In our study, the finding of lower FAs in numerous regions of the brain, including the genu, body, and splenium of the corpus callosum; posterior limb and retrolenticular part of the right internal capsule; bilateral anterior, superior, and posterior corona radiata; right posterior thalamic radiation; right sagittal stratum; right external capsule; bilateral cingulate gyrus; right fornix and stria terminalis; bilateral superior longitudinal fasciculus; and right tapetum, in A-allele carriers than in GG homozygotes is mostly consistent with previous studies showing abnormal WM connectivity with increased inflammatory processes in patients with schizophrenia. Because the highly linear structure of WM fibers confines movement in other directions, water diffusion in cerebral WM tends to be anisotropic [47,48]. FA, one of the main DTI-derived indices, provides a sensitive measurement of the degree of directionality of water diffusion along fiber tracts and provides information about microstructural changes and the integrity of the WM, including the myelin sheath, axonal membrane, and neurofibrils [49]. Reduced FA values suggest disruptions in microstructural WM integrity and axonal membranes and/or myelination [48]. Impairment of fronto-temporal functional connectivity has been suggested in patients with schizophrenia [50-53]. Supporting previous studies, the current study may indicate more severe disruption of axonal myelination in extensive fronto-temporal regions in A-allele carriers with schizophrenia than in GG homozygotes with schizophrenia. In a previous study, Aallele carriers tended to have higher levels of TNF-α expression, implying an increased inflammatory process [40]. Focusing on TNF-α, a key anti-inflammatory cytokine, our study suggests that the A allele, resulting in elevated peripheral TNF-α levels, is related to the disruption of WM integrity in schizophrenia.
We found significant associations between memory function, specifically in the verbal and visual memory domains, and the mean FAs in the corresponding brain regions after controlling for age and sex. Patients with schizophrenia who had lower mean FAs in numerous brain regions, especially in the fronto-temporal regions, produced more errors in the RKMT. Mean FAs were directly correlated with poorer verbal immediate and delayed recall, visual copying, and immediate visual recall. Neurocognitive deficits are one of the main features of schizophrenia. Among these, patients with schizophrenia tend to show impairments in verbal and visual learning. Cortical thinning of fronto-temporal regions and reduced integrity of WM tracts in fronto-temporal regions are considered in relation to impairment of neurocognitive performance [54-56]. Overlapping structural and functional dysconnectivity within the fronto-temporal regions contributes to cognitive deficits in patients with schizophrenia.
The present study had several limitations, including the relatively small sample size (n=55). Second, the data had a relatively skewed proportion of GG homozygotes (n=43) and Aallele carriers (n=12). This relatively small sample size might reduce the generalizability of these findings; nevertheless, these findings still provide some evidence supporting the hypothesis that microstructural abnormalities in fronto-temporal WM connectivity may contribute to the pathogenesis of schizophrenia and suggest a genetic disposition for differences in neurocognitive function based on the TNF-α genotype in patients with schizophrenia. Third, the direction of causality of WM changes is unknown due to the cross-sectional design of this study; nevertheless, our findings may serve as the preliminary founding of schizophrenia within the concept of the potential influence of TNF-α polymorphism and a neuroinflammatory model. Further longitudinal studies with larger sample sizes are needed to clarify the direction of causality. Fourth, our study only used the RKMT to assess neurocognitive function. Lastly, this study did not include the measurement of plasma levels of TNF-α or other inflammatory cytokines; hence, we could not evaluate whether the A-allele of the TNF-α -308 G/A polymorphism affects the plasma levels of TNF-α, although previous studies demonstrated that the A-allele of the TNF-α -308 G/A polymorphism affects the plasma level of TNF-8. Despite these limitations, this study demonstrated that the TNF-α -308 G/A polymorphism was associated with memory function impairment, specifically verbal immediate and delayed recall memory and verbal recognition memory in the RKMT, indicating that the A allele of the TNF-a -308 G/A polymorphism may be a major player in neurocognitive impairments in schizophrenia.
In conclusion, our findings suggest that the -308A allele, associated with higher levels of TNF-α expression, is correlated with poorer verbal memory function and decreased connectivity of fronto-temporal WM compared to GG homozygotes. These findings imply that impaired fronto-temporal WM connectivity might be associated with the genetic vulnerability of schizophrenia, leading to verbal memory deficits in patients with schizophrenia. The current study provides a more profound understanding of the neuropathology of schizophrenia according to the polymorphism of TNF-α, and adds more evidence related to the neuroinflammatory model of schizophrenia.
Notes
Availability of Data and Material
The data supporting the findings of this study are not publicly available due to ethical restrictions for protecting participants’ confidentiality and privacy but are accessible from the corresponding author on reasonable request with the approval of the Institutional Review Board of CHA Bundang Medical Center.
Conflicts of Interest
Minji Bang, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Minji Bang, Sang-Hyuk Lee. Data curation: Wonsuk Shin, Sra Jung, Minji Bang. Formal analysis: Naok Kang, Wonsuk Shin. Funding acquisition: Minji Bang. Investigation: Wonsuk Shin, Sra Jung, Sang-Hyuk Lee. Methodology: Wonsuk Shin, Sang-Hyuk Lee. Project administration: Minji Bang, Sang-Hyuk Lee. Resources: Minji Bang, Sang- Hyuk Lee. Software: Naok Kang. Supervision: Minji Bang. Validation: Minji Bang. Visualization: Naok Kang, Minji Bang. Writing—original draft: Naok Kang. Writing—review & editing: Minji Bang, Sang-Hyuk Lee.
Funding Statement
This study was supported by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science and ICT, Republic of Korea (grant no. NRF-2021R1C1C1012901 and NRF-2018R1D1A1B07050493). The funding sources had no role in the study design, collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.


