Systematic Review and Meta-Analysis of Damage Associated Molecular Patterns HMGB1 and S100B in Schizophrenia
Article information
Abstract
Objective
Immune system dysregulation is hypothesised to be central to the aetiopathogenesis of schizophrenia; however, the role of sterile inflammation remains unclear. Damage associated molecular patterns are key initiators of sterile inflammation and are detectable in peripheral blood.
Methods
A defined systematic search of the Web of Science, PubMed, and Scopus was performed to identify adult case-control studies published between January 1990 and June 2022. Three studies consisting of 242 cases and 83 controls met inclusion for the systematic review and meta-analysis of HMGB1 while twenty-eight studies consisting of 1,544 cases and 1,248 healthy controls were included for S100B.
Results
A significant standardised mean difference in peripheral S100B and HMGB1 concentrations was detected between cases and controls. S100B subgroup analysis determined the largest significant effect size for unmedicated individuals diagnosed with schizophrenia in the medication subgroup.
Conclusion
This study provides evidence that peripheral S100B and HMGB1 concentrations are elevated in individuals diagnosed with schizophrenia when compared with healthy controls. These results should be interpreted with caution as significant heterogeneity was present during meta-analysis of S100B in the entire sample and in sub-group analysis. The persistence of significant heterogeneity throughout subgroup analysis indicates that the current diagnostic groupings may be a barrier to understanding human behaviours and emotions.
INTRODUCTION
Schizophrenia is defined as a complex neuropsychiatric disorder affecting approximately 1% of the global population [1]. The disorder includes delusions, hallucinations, and disorganised speech as positive symptoms, while flattened affect, anhedonia, alogia, avolition, and deficits in social functioning are collectively termed negative symptoms [2,3]. Immune system alterations are intricately interlinked with neurodevelopmental [4,5], neurodegenerative [6,7], genetic [8,9], and biopsychosocial models [10,11] of schizophrenia. Multiple meta-analyses have uncovered increases in pro-inflammatory gene transcripts and protein levels including tumour necrosis factor α, interleukin 1β (IL-1β), IL-6 and C-reactive protein in the peripheral blood and post-mortem brain samples of individuals diagnosed with schizophrenia compared to healthy controls [7,12]. Maternal inflammation prior to birth [13], or a history of autoimmune disorder [14] are associated with increased risk of developing schizophrenia. Inflammation can also occur in the absence of infection. Sterile inflammation is initiated when damaged or dying cells release endogenous molecules termed damage associated molecular patterns (DAMPs), that ligate with DAMP sensing receptors and activate the innate immune response [15,16]. High mobility group box 1 (HMGB1) and S100 calcium binding protein B (S100B) are well characterised DAMPs that serve diagnostic and prognostic functions in other disorders including cancer 17 and traumatic brain injury [18].
S100B is a calcium binding protein localised in the cytosol of glial cells and astrocytes of the central nervous system [19], and both immune and non-immune cells of the peripheral nervous system [20]. S100B is important for a plethora of homeostatic activities including cell cycle progression and differentiation [21,22], calcium homeostasis [23], activation of calcium dependent enzymes [24], and microtubule assembly and disassembly of the cytoskeleton [25]. S100B is released by damaged or dying cells and interacts with the receptor for advanced glycation end products (RAGE) [26], conferring neuroprotective or neurotoxic effects on neurons in a concentration dependent manner [27]. HMGB1 is a chromatin binding protein and key epigenetic regulator that modulates the binding ability of regulatory elements such as nucleosomes and transcription factors for some genes associated with inflammation [28]. HMGB1 is expressed in all nucleated cells and remains within the nucleus under physiological conditions [29]. Damaged and dying cells release HMGB1 when the structural integrity of cells is diminished [30]. Extracellular HMGB1 interacts with a plethora of receptors including triggering receptor in myeloid cells 1, RAGE, toll-like receptor 2 (TLR2), and TLR4 to execute a number of functions pertaining to pro-inflammatory cytokine release [31], cell survival and cell death mechanisms [32,33].
The sterile immune response is critical for physiological functioning; however, chronic and sustained levels of inflammation can result in altered cellular functioning, cellular damage and death, which is hypothesised to contribute to the symptomology of schizophrenia [6]. This research aimed to quantify differences in peripheral DAMP concentrations in adults diagnosed with schizophrenia and healthy controls by conducting a systematic review and meta-analysis.
METHODS
Search criteria
A systematic search of the Web of Science, PubMed, and Scopus databases was conducted to identify relevant papers that were published between January 1990 and June 2022 using a combination of the following key search terms: (“schizophrenia” OR “schizoaffective”) AND (“HMGB1” or “high mobility Group Box 1” OR “high mobility group protein 1” OR “S100” OR “S-100” OR “S100B”). Additional articles were identified by searching bibliographical references from articles that were retrieved through the original search.
Study selection criteria
Studies identified by the initial search were screened and selected for inclusion according to the following criteria: 1) longitudinal or cross-sectional, peer-reviewed original articles, 2) article is written in English, 3) case-control studies, 4) quantitative measures of S100B and HMGB1 reported, 5) individuals diagnosed with schizophrenia in accordance with the Diagnostic and Statistical Manual of Mental Disorders, fourth or fifth edition, or World Health Organisations International Statistical Classification of Disease versions ten or eleven, and 6) sample is isolated from peripheral blood. Studies were excluded if: 1) individuals were diagnosed with comorbid neuropsychiatric disorders, 2) individuals were diagnosed with a chronic inflammatory, neurological, or autoimmune disorder, 3) individuals under the age of 18 were included, and 4) arithmetic mean and standard deviation for measures of S100B and/or HMGB1 were not reported or could not be extracted from the article.
Data extraction
Data pertaining to age, sex, concentration of peripheral S100B and/or HMGB1, neuropsychiatric diagnostic method, quantitative assay used, sample location, medication usage, psychopathological state, and medical history were collected where applicable for both the control and schizophrenia cohorts. In the case of longitudinal studies that included repeated blood draws and quantitative measurement of S100B and HMGB1, the values reported from the initial blood draw were included. In the case where the same patient cohort samples were published in multiple journal articles, the first article to appropriately report relevant measures was included, with all other articles using identical samples excluded. When the sample cohort of individuals diagnosed with schizophrenia was further divided into subpopulations and no information for the entire schizophrenia group was reported, the data from the subpopulations was pooled to generate a pooled mean and standard deviation. Quantitative data that was reported in alternative units was converted to ng/L for S100B and ng/mL for HMGB1 to allow comparison. When the arithmetic mean and standard error (SE) of the mean was not reported, the following calculation as used to convert the SE of the mean to standard deviation: standard deviation=(SE of the mean)×(√n) [34]. The online tool WebPlotDigitizer (Pacifica, CA, USA) was used to extract data reported in graphic form only.
Statistical analysis
A meta-analysis was conducted for the thirty studies included in the systematic review of S100B. The standardised mean difference between individuals diagnosed with schizophrenia and controls served as the main outcome measure, while the conservative random effects model was fitted to the data to account for inter-study variance [35]. The effect size was reported as Hedges’ g value with 95% confidence intervals (CIs) [36]. Statistical heterogeneity was measured using Hedges estimator and reported as tau2 [36], the Q-test for heterogeneity [37], and the I2 statistic [38]. Subgroup analysis was performed to explore possible causes of heterogeneity. Publication bias was measured using the Fail-Safe N [39], Begg and Mazumdar’s rank correlation [40], Egger’s regression [41], and trim and fill number of Studies [42]. The statistical analysis software jamovi (V1.6) [43] was employed to conduct all statistical analysis.
Data reporting
This systematic review and meta-analysis were conducted in accordance with the most recent version of the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines [44].
RESULTS
Characteristics of included studies
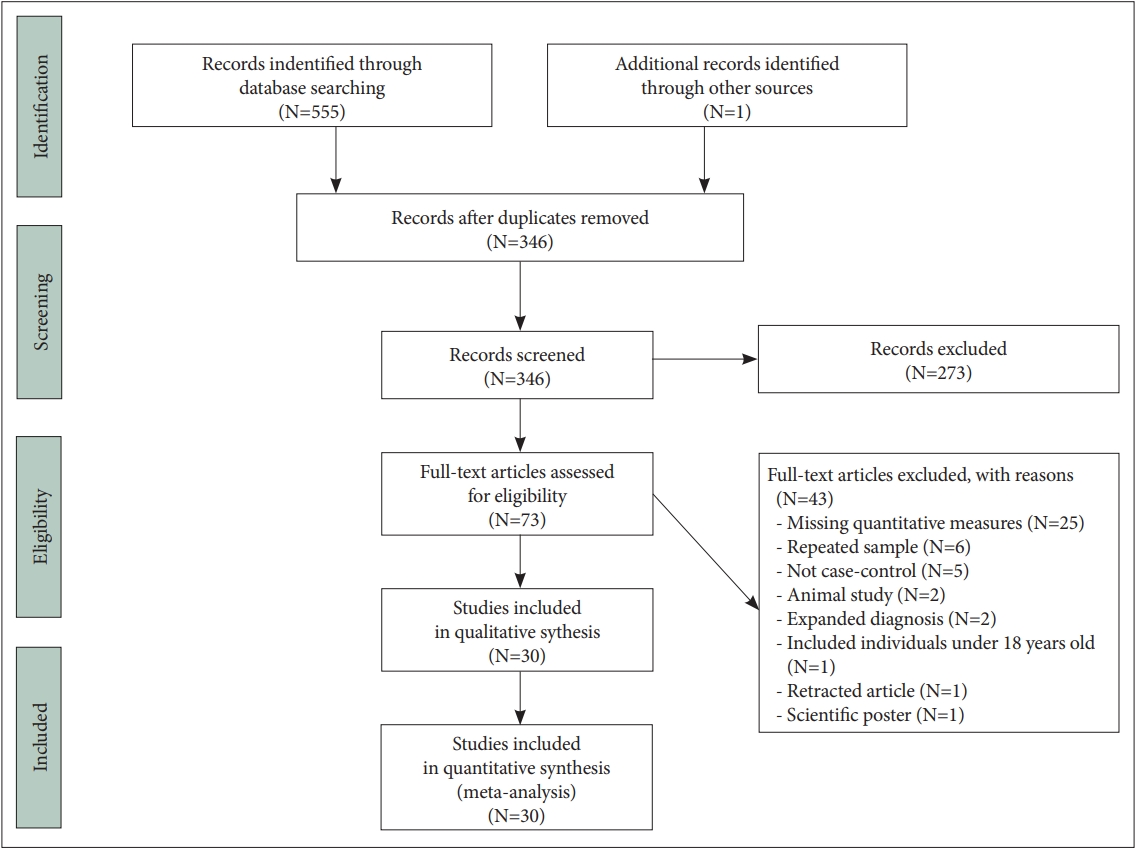
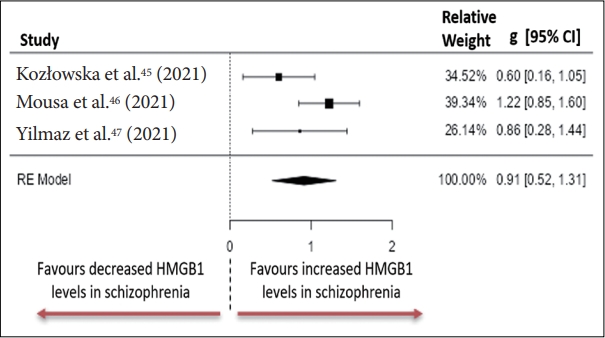
The literature search identified 555 potentially relevant publications, of which 30 articles satisfied the inclusion criteria (Figure 1). One article was applicable for inclusion in both S100B and HMGB1 analysis [45]. Two other articles were suitable for inclusion in the HMGB1 group [46,47] and 28 articles were applicable for inclusion in the S100B group which are systematically reported in Suppletmentary Table 1 (in the online-only Data Supplement). Quantitative analysis of HMGB1 concentration was performed on serum samples from participants using an ELISA assay for all three studies. Kozłowska et al. [45], (2021), measured clinical symptom severity using the Scale for assessment of Negative Symptoms (SANS), and Calgary Depression Scale for Schizophrenia (CDSS), while Mousa et al. [46], (2021) and Yilmaz et al. [47], (2021) used the Positive and Negative Symptom Scale (PANSS).
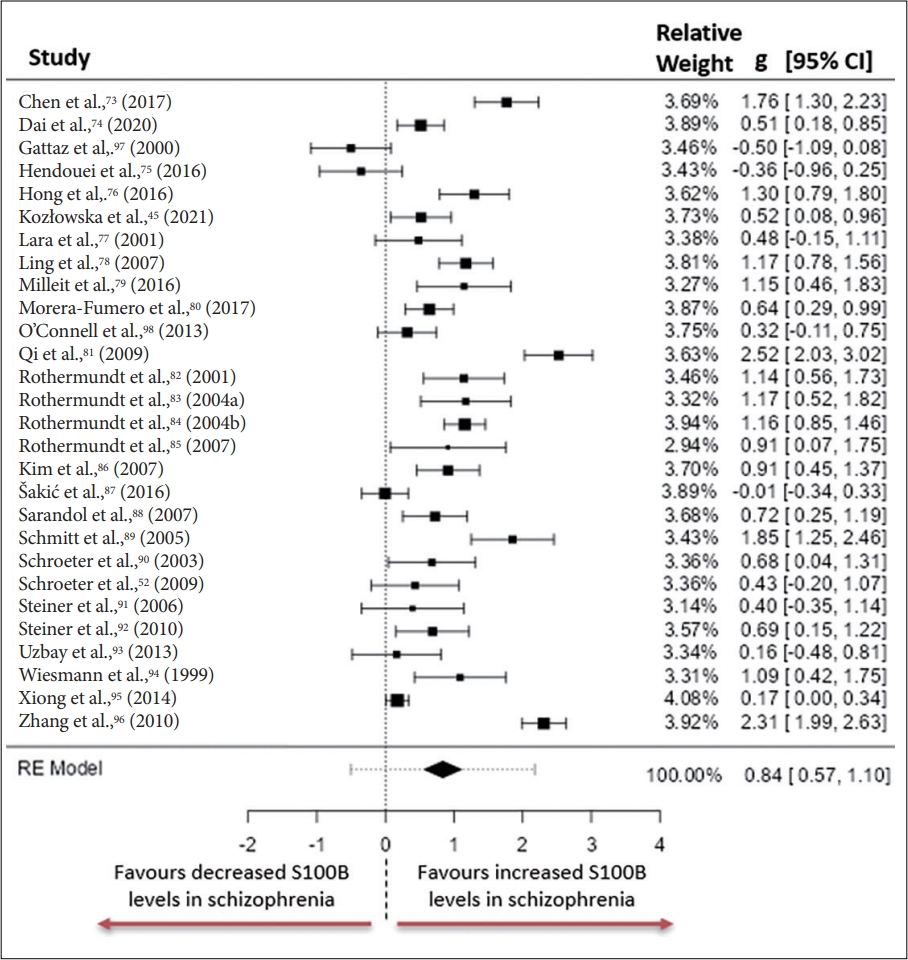
In total twenty-eight studies consisting of 1,544 individuals diagnosed with schizophrenia and 1,248 healthy controls were included in the systematic review and meta-analysis of S100B. The characteristics of individuals included is illustrated in Figure 2 and reported in Supplementary Table 1 (in the online-only Data Supplement). Ten studies included individuals who were exclusively unmedicated, ten studies included both individuals who were medicated and individuals who were not medicated, while the remaining eight studies included individuals who were all actively taking medication. Twenty studies recruited inpatients exclusively, while only three studies recruited only outpatients. A further two studies recruited both inpatients and outpatients, while three studies did not mention if individuals were receiving inpatient or outpatient care. The psychopathological state of individuals was characterised using the PANSS in 22 studies, the Brief Psychiatric Rating Scale (BPRS) [48] in six studies, the SANS was used in three studies, the Scale for assessment of Positive Symptoms (SAPS) [49] in two studies, the Negative Symptom Rating Scale (NSRS) [50] in one study and the CDSS in one study.
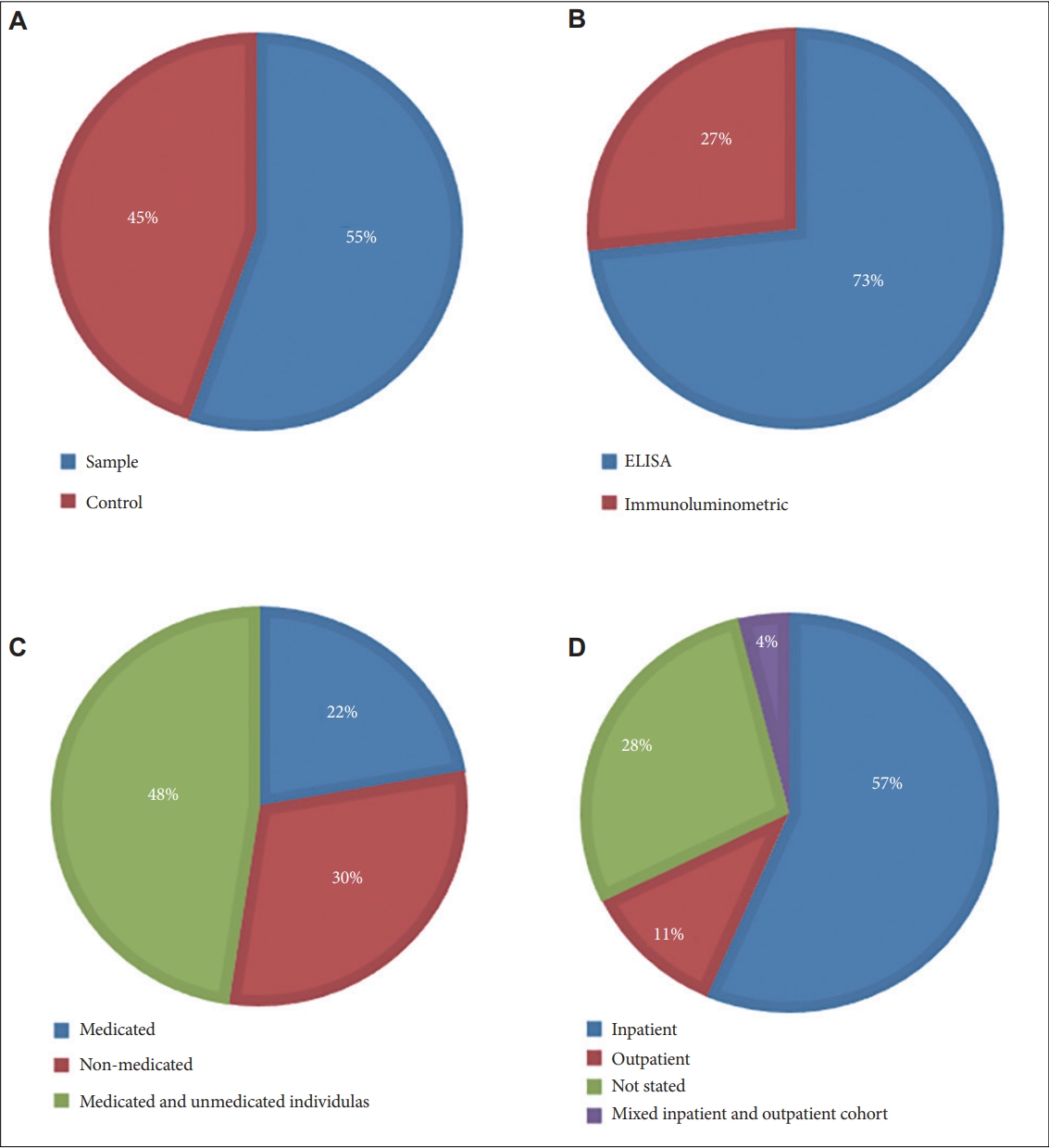
Pie charts illustrating characteristics of individuals included in the S100B systematic review and meta-analysis. A: Case and control. B: Assay. Pie charts A and B are representative of the whole population of individuals. C: Medication status. D: Patient setting. Pie charts C and D are exclusively representative of individuals diagnosed with schizophrenia. Unmedicated individuals described in pie chart C represents individuals who were not taking antipsychotic medication for at least one week prior to study commencement. ELISA, enzyme linked immunosorbent assay.
Meta-analysis of HMGB1
Individuals diagnosed with schizophrenia had significantly higher peripheral blood concentrations of HMGB1 compared to healthy controls. The observed standardised mean differences between the groups ranged from 0.6023 to 1.2237 with all the estimates having positive directionality (Figure 3). The estimated standardised mean difference between case and control groups determined from the random effects model was g=0.9145, 95% CI (0.5203 to 1.3087) an effect size that differed significantly from zero (z=4.5470, p<0.001). Heterogeneity was measured using: Tau2=0.0661 (SE=0.1221), I2=54.72% and Q=4.496, p=0.106. Analysis of studentised residuals uncovered that all studies had a value smaller than ±2.3940. Publication bias was assessed and reported as: fail=safe, n=51.00, p<0.001; Begg and Mazumdar rank correlation=-0.33, p>0.999; Egger’s regression=-0.494, p=0.621; trim and fill number of studies=2.00.
Whole group meta-analysis of S100B
Similarly, S100B concentrations were significantly higher in peripheral blood of individuals diagnosed with schizophrenia compared to healthy controls. The observed standardised mean differences between the groups ranged from -0.5027 to 2.5247, with only three of the twenty-eight studies not reporting a positive effect (Figure 4). The estimated standardised mean difference between case and control groups determined from the random effects model was g=0.8363, 95% CI (0.5688 to 1.1038) with this effect size differing significantly from zero (z=6.1282, p<0.001). Heterogeneity was measured using: Tau2=0.4486 (SE=0.1416), I2=90.09% and Q=300.336, p<0.001. Analysis of studentised residuals uncovered that all studies had a value smaller that ±3.1237. Publication bias was assessed and reported as: fail-safe, n=3467.00, p<0.001; Begg and Mazumdar rank correlation=0.021, p=0.891; Egger’s regression=-0.238, p=0.812; trim and fill number of studies=6.00.
Subgroup meta-analysis of S100B
Subgroup analysis was performed to assess interstudy heterogeneity and determine the influence of potentially confounding factors on the standardised mean difference between cases and controls, through stratification of participants. The sample (serum or plasma) used to quantitatively measure S100B, the assay used to measure S100B, the medication status of patients, and patient care status (inpatient or outpatient) were the subgroups used, with the standardised mean difference determined from the random effects model. The mean effect size was reported as hedges g with 95% CIs, and interstudy heterogeneity reported as I2. Subgroup analysis is summarised in Table 1.
DISCUSSION
To our knowledge, this is the first meta-analysis to assess peripheral HMGB1 and the third such meta-analysis to compare peripheral S100B concentrations in adults diagnosed with schizophrenia and healthy controls [51,52]. This meta-analysis detected significantly elevated peripheral S100B and HMGB1 concentrations in adults diagnosed with schizophrenia compared to healthy controls. An overrepresentation of individuals diagnosed with schizophrenia using inpatient services (Figure 2) suggests that this study is more representative of individuals in an acute phase of illness compared to the whole population of individuals diagnosed with schizophrenia.
In agreement with previous meta-analyses, S100B concentrations are significantly elevated in the peripheral blood of adults diagnosed with schizophrenia compared with healthy controls [51,52]. Novel to this meta-analysis was differential S100B concentrations detected in the peripheral blood of medicated and unmedicated subgroups of individuals diagnosed with schizophrenia. The subgroup of unmedicated individuals diagnosed with schizophrenia had the largest standardised mean difference between case and control populations, in the medication subgroup, while medicated individuals diagnosed with schizophrenia had a lower but significant standardised mean difference between case and control groups. This suggests that antipsychotic medication may influence the immune system and partially elicit therapeutic effects through modulation of immune related pathways. At present, a distinct lack of clinical research examining the influence of antipsychotic medication on peripheral S100B concentrations exists. An in-vitro study has provided evidence that both typical and atypical antipsychotic medication can reduce S100B release from neuronal cell lines [53]. Further research is needed to understand if this effect translates to clinical populations. Studies on drug naïve individuals have reported significantly reduced IL-1β and IL-6 levels in the months following treatment with antipsychotic medication [54,55]. A consensus remains to be reached with longitudinal studies reporting that levels of pro-inflammatory cytokines return to their drug naïve baseline or even higher concentrations over time [56-59].
The triangulation of available evidence suggests that elevated peripheral S100B and HMGB1 levels are characteristic of schizophrenia. Physiological concentrations of S100B and HMGB1 are critical for homeostasis. However, sustained supraphysiological expression can induce altered cellular functioning, cellular damage and death, which is hypothesised to contribute to the symptomology of schizophrenia [6,60,61]. These DAMPs are also known to engage with pattern recognition receptors and therefore induce further inflammation [16]. Future longitudinal research exploring their potential causative or consequential roles in schizophrenia is required.
High levels of statistical heterogeneity were a major limiting factor of this study and were also noted in previous meta-analyses [51]. Understanding the sources of interstudy heterogeneity is of paramount importance as the validity of meta-analyses is dependent on this. Subgroup analysis was employed to assess potential contributors to the whole group heterogeneity but failed to reduce heterogeneity despite segregating participants based on potential effect modifiers and confounds including medication status, assay used, sample source and patient treatment status. It is possible that other potentially confounding factors that were not controlled for in this study including body mass index levels [62], diet [63], smoking habits [64], and exercise levels [65] may have contributed to the observed heterogeneity.
Recent criticisms have highlighted the broad and heterogeneous grouping of individuals into diagnostic categories that encompass a vast number of overlapping symptoms, while failing to account for symptom severity [66,67]. Symptomology is hypothesised to more likely reflect neurobiological alterations as opposed to classical diagnostic groupings, indicating that heterogeneity introduced by clinical diagnosis is a barrier to understanding the neurobiological mechanisms underpinning dominant symptomology of neuropsychiatric disorders [68]. Efforts are underway to uncover and integrate neurobiological markers of neuropsychiatric disorders with more traditional diagnostic approaches [69]. Finally, the absence of an independent assessor or second person verification step may increase the risk of missing potentially relevant information.
The disparity between health of individuals diagnosed with schizophrenia and that of the general population has widened in recent decades, despite the advent of first and second generation antipsychotics and continuing research [70,71]. Understanding the role of current diagnostic groupings in contributing to clinical heterogeneity, and its validity as a conceptual model for which to study human thoughts, behaviours, and emotions should be a research priority as the gap between the health of individuals diagnosed with schizophrenia and the general population widens.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2022.0173.
Systematic review of S100B and HMGB1 levels in individuals diagnosed with schizophrenia and healthy controls
See Supplementary Materials for versions that show errors and corrections.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: all authors. Data curation: Michael Mackey, Declan P. McKernan. Formal analysis: Michael Mackey, Declan P. McKernan. Investigation: all authors. Project administration: Laurena Holleran, Gary Donohoe, Declan P. McKernan. Software: Michael Mackey, Declan P. McKernan. Supervision: Declan P. McKernan. Validation: Gary Donohoe, Declan P. McKernan. Visualization: Michael Mackey, Declan P. McKernan. Writing—original draft: Michael Mackey, Declan P. McKernan. Writing— review & editing: Laurena Holleran, Gary Donohoe, Declan P. McKernan.
Funding Statement
None