Confounding by Indication in Studies of Selective Serotonin Reuptake Inhibitors
Article information
Abstract
Selective serotonin reuptake inhibitors (SSRIs) are used worldwide as the first-line pharmacological treatment for depression. Although SSRI use can increase the risk of suicide, fractures, and infertility, the nature of these relationships is controversial. This review reports confounding by indication and confounding by severity for SSRI side effects in previously published observational studies. The PubMed and Google Scholar databases were searched for English-language articles published from 2005 to 2022. SSRIs are often prescribed for depressive symptoms, and depression is associated with an increased risk of side effects. Therefore, confounding by indication, whereby patients are selected for a particular treatment depending on their diagnosis or severity of illness, may lead to erroneous treatment conclusions, resulting in an adverse outcome. The side effects of SSRIs that can be considered due to confounding by indication or severity include suicide, fractures, infertility, atrial fibrillation, stroke, autism spectrum disorder, and congenital malformation. When prescribing SSRIs for depression, physicians must consider confounding by indication and severity in the management of side effects. In addition, medication discontinuation should be carefully considered when side effects occur during the treatment.
INTRODUCTION
Depression is the most prevalent psychiatric disorder in the world, affecting 4.4% of the global population [1]. Despite an array of treatment modalities, depressive disorders are difficult to manage due to several factors, including relatively high relapse rates while undergoing treatment and unfavorable side effect profiles of the available medications [2,3].
Selective serotonin reuptake inhibitors (SSRIs) are used worldwide as the first-line pharmacological treatment for depression [4]. However, SSRI use is associated with several side effects including loss of appetite, weight loss, drowsiness, dizziness, fatigue, headaches, increased suicidal thoughts, nausea/vomiting, sexual dysfunction, and increased risk of cardiovascular and cerebrovascular events [5-7].
Serotonin receptors comprise at least seven classes, which are further divided at the sub-receptor level [8]. These receptors mediate various functions, including sleep, appetite, and sexual function, and symptoms, such as depression and anxiety. By increasing the inhibition of serotonin reuptake, a higher amount of the neurotransmitters is available to interact with any of these receptors or subtype receptors. Therefore, most side effects of SSRIs are dose-related and are attributed to serotonergic effects [8]. Moreover, depression, an indication for SSRIs, may act as a confounding factor for SSRI side effects [9].
Confounding by indication is defined as a bias in the treatment-intended outcome relationship due to the clinical reasons for treatment (Figure 1), with the indication for treatment based on physician and patient perceptions of disease severity and prognosis, including the presumed therapeutic effect of the intervention [10]. Confounding occurs because the treatment and indication are closely correlated, and a greater intensity of the indication results in a higher rate of the undesired outcome. Such confounding is typically negative and serves to dilute or reverse the effects of the treatment [11]. Confounding by severity is also considered a type of confounding by indication, in which the disease that is the indication and severity of the disease are potential confounders [12]. If the disease is considered, but its severity is not, there is a possibility of residual confounding. Therefore, the disease stage and its corresponding severity and complications are important.
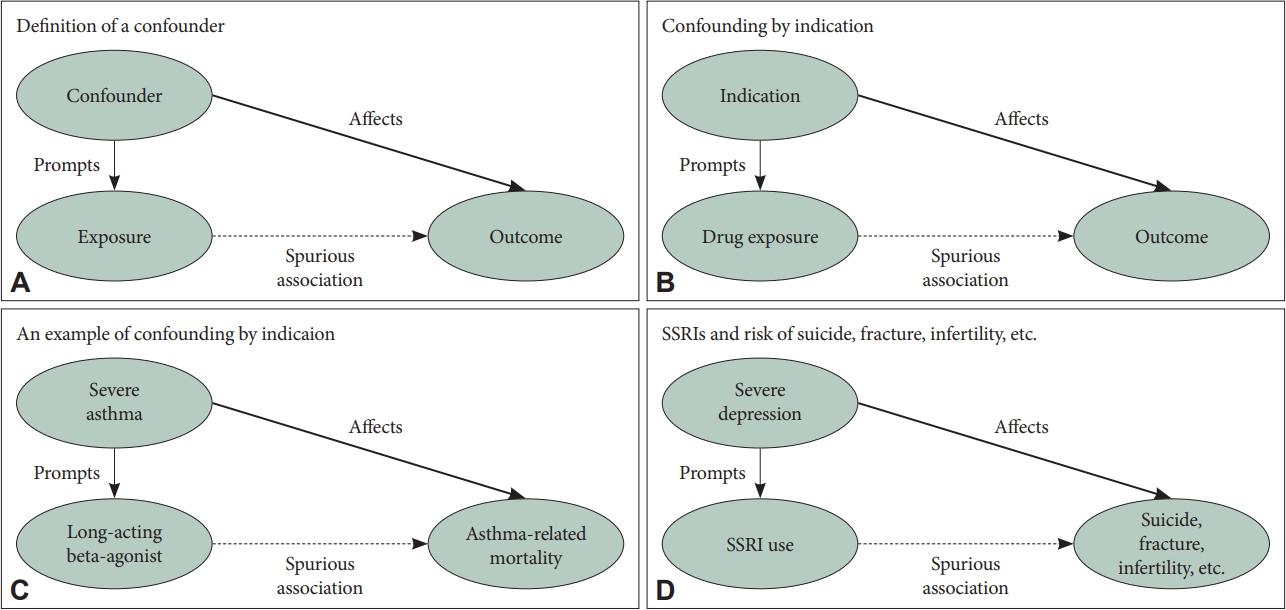
Relationships between the confounder, exposure, and outcome. A: The confounder involves an extraneous factor that affects both the exposure and outcome. In the presence of an unadjusted confounder, spurious associations between the exposure and outcome can occur as the exposure can be artificially linked to the outcome through the confounder. B: Confounding by indication is a form of confounding that is unique to observational studies on the effects of drugs. The indication for prescribing a drug affects both the drug exposure and outcome, and, therefore, serves as a confounder to create the spurious association between the drug exposure and outcome. C: An example of confounding by indication is shown. Patients with severe asthma (the indication) are more likely to be prescribed long-acting beta-agonists (drug exposure). At the same time, severe asthma (the indication) also affects a higher risk of asthma-related mortality (outcome). Therefore, if the severity of asthma is not adjusted for in observational studies, spurious results may occur, suggesting that the use of long-acting beta-agonists can lead to a higher risk of asthma-related mortality. D: An example of confounding by indication in a patient with severe depression is shown. SSRIs are prescribed for severe depression, which can potentially serve as a confounder and require further adjustment. Patients with severe depression are more likely to be prescribed SSRIs, and have higher risks of suicide, fracture, infertility, etc. SSRI, selective serotonin reuptake inhibitor.
When selecting a therapeutic drug, side effects have a greater impact than efficacy in terms of compliance; therefore, side effects of the primary disease and its severity should be considered along with the side effects due to medications [9,13]. In this review, confounding by indication and severity of SSRI side effects in observational studies are systematically analyzed.
METHODS
The PubMed (available at http://www.pubmed.ncbi.nlm.nih.gov) and Google Scholar (available at https://scholar.google.com) were searched for studies published between 2005 and 2022 using the following terms: “SSRI” cross-referenced with “side effects,” “confounding bias,” “by indication,” and “by severity.” The eligibility criteria for selecting studies were as follows: 1) Cohort studies or nested case-control studies, 2) studies that investigated the association between the use of SSRIs and the risk of each side effect, 3) studies that reported outcome measures with a relative risk or odds ratio and 95% confidence interval, and 4) studies that were available in English.
RESULTS
A total of 145 articles were identified. After screening of the title and abstract, the full text of 28 articles was extensive reviewed by two reviewers. Finally, 16 studies—one study reporting suicide, one study reporting fractures, five studies reporting infertility, one study reporting atrial fibrillation (AF), one study reporting stroke, five studies reporting autism spectrum disorder (ASD), and two studies reporting congenital malformation—were included (Table 1).
DISCUSSION
Suicide
The potential for SSRIs to increase suicide is concerning [14]. However, evidence of an increased rate of completed suicides associated with SSRI use is limited and contradictory [15,16]. Observational studies have reported that SSRI use significantly increases the risk of completed or attempted suicide in adolescents, significantly decreases the risk of completed or attempted suicide in adults, and has a significant protective effect in patient aged ≥65 years [17,18].
Data reported in previous observational studies might have been compromised by confounding by severity or indication. Identification of associations between a treatment and an outcome when the outcome itself is strongly associated with the condition being treated is limited in observational studies. Confounding by indication, whereby patients are selected for a particular treatment based on the diagnosis, or severity of illness, may lead to erroneous treatment conclusions, resulting in an adverse outcome [19]. Medical treatments are typically more likely to be prescribed to more severely ill patients who are already at a higher risk of suicide. In addition, SSRI treatment has a low risk of toxic effects from acute overdosing; therefore, SSRIs are more likely to be prescribed than are more toxic agents, such as tricyclic antidepressants (TCAs), for patients at an increased risk of suicide.
In a retrospective cohort study of 57,361 patients conducted in New Zealand, 26 suicides were reported within 120 days of antidepressant treatment [20]. No association between SSRI use and suicide was observed after adjusting for the confounding effects of age, sex, and pre-existing depression/suicidal ideation. These findings suggest that clinicians are preferentially prescribing SSRIs, especially newer SSRIs, to patients with a higher risk of suicide and prescribing TCAs to patients for indications other than depression [20]. Therefore, pre-existing depression is a significant confounding factor in observational studies of the association between antidepressants and suicide.
Fractures
The association between daily SSRI use and fragility fractures among older patients is unclear as it is based on data from observational studies rather than those from prospective trials. A causal relationship between SSRI use and fragility fractures is not supported by sufficient data; therefore, to label SSRIs as a secondary cause of osteoporosis is premature. A Swedish register-based study reported an increased risk of fragility fractures among patients receiving SSRIs; however, in the adjusted analyses, the highest risk of fracture was observed 16–30 days before initiating antidepressants [21]. Moreover, antidepressants, including SSRIs, cannot increase the risk of hip fracture before treatment initiation; therefore, the increased risk of fracture is likely due to the illness for which the drug is prescribed.
Potential confounding by indication should be considered in any observational study on the effects of drugs [22]. SSRIs are often prescribed for depressive symptoms, and depression is associated with an increased risk of fractures [23]. Depression may directly lower the bone mineral density (BMD) via several pathways. For example, persistently elevated plasma cortisol levels are associated with clinical depression [24]. Michelson et al. [24] reported that increased cortisol secretion in women might lead to decreased BMD. Elevated plasma cortisol levels in depression are associated with hypothalamic–pituitary–adrenal (HPA) axis disturbances [25,26]. Lower BMD and osteoporotic fractures involve complex mechanisms associated with hypothalamic dysfunction that are not fully elucidated, including hypercortisolism and hypogonadism [25]. Thus, through these hypothalamic disturbances, bone metabolism may be altered in patients with depression. Depression may also indirectly affect behaviors that lead to an increased risk of decreased proximal femoral BMD [27,28]. For example, depression is associated with increased smoking and alcohol consumption and decreased physical activity [29-32].
Infertility
SSRIs often lead to decreased libido, erectile dysfunction, and delayed ejaculation in men and genital anesthesia, loss of lubrication, and anorgasmia in women [33]. In addition, SSRIs may alter the release of pituitary hormones, which adversely affects ovulatory function in women and spermatogenesis in men, further contributing to the association between psychological stress and reduced fertility [34,35].
The impact of SSRIs on female fertility is less clear than that on male fertility, which may be due to the fact that fertility parameters in women cannot be measured as easily as those in men. Few studies have examined the effect of SSRIs on fertility or fecundability in the female population prior to seeking an infertility workup. Casilla-Lennon et al. [35] conducted a prospective cohort study including 957 women without a history of infertility who expressed a desire to conceive. In this previous study, 92 patients who were prescribed antidepressants were less likely to become pregnant than those who were not prescribed antidepressants, although the difference was insignificant. However, depression was assessed only at baseline, and the authors did not control for the symptoms of depression in this previous study. Furthermore, an SSRI prescription was used as a surrogate measure of active disease, rendering it more difficult to distinguish the effects of the disease from those of the medication.
One cohort study including women trying to conceive reported an inverse association between depressive symptoms and fertility, although antidepressants were not found to influence the probability of conceiving [36]. Similarly, Hernandez-Nieto et al. [37] reported no significant effects of SSRIs on the rate of embryonic aneuploidy, implantation, clinical pregnancy, pregnancy loss, or multiple pregnancy in women who underwent in vitro fertilization. In addition, Andersen et al. [38] reported an increased risk of miscarriage in women who were administered antidepressants during the first trimester compared to that in women who were not. However, a similar increased risk of miscarriage in women who discontinued the antidepressant prior to conceiving was also observed, suggesting confounding by indication.
Although several studies have evaluated the effects of psychotropic medication use on semen quality [39], only one study has evaluated the association between male psychotropic medication use and directly measured fertility outcomes (fecundability) [40]. In a cohort study on fecundability, current use of SSRIs was associated with slightly reduced fecundability, although this result was not significant. In a cohort study on sperm quality [39], a history of depression was associated with a 4.3-fold increase in the risk of low semen volume. Recent psychotropic medication use (including SSRIs use) was associated with worse semen quality; however, this association was confounded by a history of depression.
It has been proposed that depression decreases fecundability indirectly by reducing energy, libido, or self-esteem or by causing sexual dysfunction [41]. Psychological stress has been linked to dysregulation of the HPA axis, which may cause reproductive organ dysfunction and potentially result in infertility. Infertility is associated with elevated levels of psychological stress, which may lead to or accentuate this phenomenon [34,42]. Patients with depression also tend to have problems with obesity, alcohol consumption, and tobacco use [43], which affect fertility [44].
Atrial fibrillation
SSRIs result in elevated serum serotonin levels. Serotonin promotes calcium overload, which may trigger focal atrial extrasystoles and increase the risk of AF [45,46]. In contrast, SSRIs can correct the imbalance of proinflammatory cytokines in patients with depression [47-49], which may reduce the risk of depressioninduced AF. Thus, the increased risk of AF due to SSRI use is controversial.
In a nationwide Danish register-based study [50], 785,254 adults were prescribed antidepressants, including SSRIs. After adjusting for sociodemographic and clinical confounding factors, SSRI use was associated with a 3-fold risk of AF during the first month after initiating antidepressant medication. However, this risk gradually decreased 2–6 and 6–12 months after antidepressant medication initiation. Importantly, the risk of AF was the highest 1–15 days and 16–30 days before the initiation of antidepressant medication. Therefore, antidepressant use is simply a marker for the risk of AF. The indication for the antidepressant or characteristics of patients for whom antidepressants are prescribed may be the factor(s) resulting in an increased risk of AF. The risk factors of AF may be related to depression and may be state dependent as the risk progressively decreased over time with the resolution of the indication for which the antidepressant was prescribed.
The association between depression and AF has been confirmed in several studies [51,52]. Depression is closely related to the dysregulation of the HPA axis and inflammation. Hyperactivation of the HPA axis may induce the consistent release of cortisol, which serves as a marker for cortisol resistance [53]. Cortisol resistance stimulates immune activation and increases the expression of proinflammatory cytokines, including interleukin (IL)-2, IL-6, IL-12, and tumor necrosis factor-α. These cytokines act on the brain, resulting in symptoms of depression in susceptible populations [54,55], and lead to systemic inflammation. The involvement of the HPA axis indicates that depression is often accompanied by hypertension, metabolic syndrome, and obesity [56-58], which aggravate oxidative stress and inflammation in the body. Systemic inflammation increases the risk of AF by changing the electrophysiology (calcium flowing), conduction, and structural substrates of the atrium [59,60]. In addition, depression may alter the sympathetic and parasympathetic balance, resulting in a decreased arrhythmic threshold [61], affecting the atrial conductivity and structural integrity [54].
Stroke
The relationship between SSRI use and stroke is controversial, as several studies have reported an increased risk, while, few studies have reported a decreased risk or a neutral effect [62].
SSRI administration may increase the risk of bleeding, including the risk of hemorrhagic stroke, due to their antiplatelet effects [63]. SSRI-induced serotonergic activation may also cause ischemic stroke via arterial vasoconstriction [63]. However, depression, an indication for SSRIs, may contribute to stroke through various mechanisms. Depression has neuroendocrine effects (sympathetic nervous system activation, dysregulation of the HPA axis, and platelet aggregation dysfunction) and immunological/inflammation effects [64,65], which may affect the risk of stroke. In addition, depression is positively associated with C-reactive protein, IL-1, and IL-6 levels in clinical and community populations, and these inflammatory factors are associated with an increased risk of stroke [66,67]. Furthermore, depression is associated with poor health behaviors (such as smoking, physical inactivity, poor diet, and lack of medication compliance) and obesity [68,69], which may increase the risk of stroke. In previous studies, adjustment for smoking or body mass index somewhat attenuated the association between depression and stroke, suggesting that smoking and obesity might confound or mediate the association between depression and stroke [70]. Finally, depression is correlated with major comorbidities that are major risk factors for stroke, such as diabetes and hypertension [71,72].
In a nested case-control study, patients actively using SSRIs had an increased risk of ischemic stroke compared to that in patients who did not use antidepressants [62]. However, after adjusting for depression or a history of antidepressant use, no increased risk was found. In addition, patients actively using SSRIs had a decreased risk of stroke compared to that in patients using other antidepressants (non-SSRIs and non-serotonin antagonist and reuptake inhibitors). This previous study suggested that the positive association between SSRIs and stroke could be explained by several methodological factors, including confounding by the indication. However, there are limited studies on confounding by indication, and more studies are needed to confirm this association.
Autism spectrum disorder
SSRIs are the most common antidepressants used to prevent maternal depression during pregnancy. However, the safety of these medications is not well-established. SSRIs cross the placenta and are transferred to the fetus. Several studies have been conducted to assess the causality of the association between prenatal exposure to SSRIs and risk of ASD.
In previous studies, the risk of ASD in the offspring of mothers with SSRI exposure during pregnancy was significantly higher than that in the offspring of mothers without SSRI exposure [73,74]. The results of previous studies on the risk of ASD in the offspring of mothers with SSRI exposure during pregnancy are congruent and suggest a biological mechanism underlying ASD development, serotonin metabolism dysfunctions, and serotonergic changes [75,76]. However, these findings of observational studies are difficult to interpret due to limitations in unmeasured confounding, including confounding by indication and severity of depression, which is inherent to this type of research.
Gidaya et al. [77] have reported that SSRI use prior to conception is associated with a significant increase in the risk of ASD in the offspring. The risk of ASD in offspring of mothers exposure to antidepressant during pregnancy is not significantly higher than that in offspring of unexposed women with a history of affective disorder [78,79]. In a sibling analysis conducted by Sørensen et al. [80], no difference in the risk of ASD was found between siblings exposed to SSRIs in utero and those who had not been exposed to SSRIs in utero.
A cohort study that included a nested sibling case-control analysis reported that the risk of ASD was higher in offspring of mothers with treated depression and untreated depression than in offspring of mothers with no history of depression or antidepressant use (i.e., not exposed to SSRIs) [81]. In addition, the risk of ASD was not increased in offspring of women who were administered SSRIs for other indications. Additional analyses to assess the effects of the severity of depression suggested that the risk of ASD in offspring increased with the increasing severity of depression, not with the antidepressant treatment itself [81]. These findings indicate that an increased risk of ASD after antidepressant exposure during pregnancy is likely to lie with correlates of maternal depression and the severity there of rather than with the use of medications to treat the depression.
Congenital malformations
SSRIs cross the placenta and can affect specific cells and tissues during embryogenesis, which may result in congenital malformations, especially cardiac malformations [82]. The associations between SSRI use during pregnancy and the risk of congenital malformations in offspring are controversial. Depression is associated with adverse pregnancy outcomes and health behaviors [83]. Therefore, maternal depression may increase the risk of congenital malformations in infants [84].
Previous studies have suggested that the association between SSRI use and congenital malformation, especially congenital heart disease (CHD), can be explained by a confounding bias. A Danish study reported a higher risk of CHD in the offspring of women administered SSRIs in the first trimester of pregnancy and in those who discontinued SSRI use during pregnancy, suggesting confounding by the underlying depression or factors associated with depression [85]. An American study reported that controlling for co-exposures to other medications reduced the risk of CHD in an analysis limited to women with depression [86]; although the effects of SSRIs cannot be completely distinguished from those of psychiatric illness; such estimates are likely to be influenced by confounding by indication.
Depression increases the risk of self-injurious or suicidal behaviors in mothers and may also contribute to inadequate self-care and poor compliance with prenatal care. Women with depression often present with decreased appetite, resulting in lower-than-expected weight gain during pregnancy, which is associated with negative pregnancy outcomes [87]. In addition, pregnant women with depression are more likely to smoke, consume alcohol, or use illicit drugs [87], which increases the risks to the fetus. The physiological mechanisms by which symptoms of depression may affect neonatal outcomes are unclear. However, increased serum cortisol and catecholamine levels, which are typically observed in patients with depression, may affect placental function by altering uterine blood flow and inducing uterine irritability [88]. Dysregulation of the HPA axis, which is associated with depression, may also have a direct effect on fetal development [89].
Conclusion
When prescribing SSRIs to patients with depression, physicians should consider confounding by indication or severity in patients with side effects. In addition, the management of side effects, including discontinuation of SSRIs, should be conducted carefully in patients with depression.
Notes
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Sung Man Chang. Investigation: Jimin Lee, Sung Man Chang. Writing—original draft: Jimin Lee. Writing—review & editing: Jimin Lee, Sung Man Chang.
Funding Statement
None

