Effectiveness of Clonidine in Child and Adolescent Sleep Disorders
Article information
Abstract
Objective
We aimed to investigate the improvement in sleep quantity and quality when clonidine was used in children and adolescents with insomnia. We also examined how sociodemographic characteristics such as age, sex, underlying psychological problems, and levels of depression and anxiety affected the effect of clonidine.
Methods
We retrospectively reviewed outpatients aged 6 to 24 who took clonidine due to insomnia from September 2019 to September 2021 at the Department of Psychiatry at Eunpyeong St. Mary’s Hospital of Catholic University. We used the Pittsburgh Sleep Quality Index (PSQI), Children’s Depression Inventory (CDI), and State-Trait Anxiety Inventory (STAI) for our study.
Results
A total of 62 participants were included in our study (34 females, mean age 13.94±4.94 years). After using clonidine, there was a significant decrease in PSQI components 1, 2, and 5, especially PSQI component 2. There was a greater decrease in sleep latency when clonidine was used in females, those aged between 13 and 24, those with mood/anxiety disorder or attention-deficit/hyperactivity disorder, those whose sleep latency exceeded 60 minutes at baseline, and those who used clonidine for more than 14 days. Those with higher STAI-Trait scores and CDI scores at baseline showed less improvement in total PSQI scores.
Conclusion
Considering that there are currently no Food and Drug Administration-approved sleep drugs for children and adolescents and no apparent difference in efficacy and safety among sleep drugs, we demonstrated that treatment with clonidine might be a good approach to improve sleep quality and quantity for children and adolescents.
INTRODUCTION
Sleep problems are very common among children and adolescents worldwide, and it seems that approximately 25% of all children experience several types of sleep problems during childhood [1]. In the LIFE Child study, 22.6% of the 4- to 9-year-old children and 20.0% of the 10- to 17-year-old adolescents showed significant amounts of sleep-related problems [2]. A recent study assessing sleep-related problems in 7.3–14.9-year-old children reported that 72.7% of the children had sleep disorders [3,4]. Although behavioral therapy is preferred as the initial treatment for sleep disorders in children and adolescents, pharmacologic interventions also play a considerable role for them [5,6]. However, there are currently no Food and Drug Administration (FDA)-approved sleep medications for children and adolescents; thus, many clinicians have prescribed medications for “off-label” indications [7].
Among the “off-label” sleep medications for children and adolescents, there has been increasing interest in the use of clonidine as a sleep medication, especially for patients with attention-deficit/hyperactivity disorder (ADHD), developmental delays, autism spectrum disorders (ASD), and genetic syndromes [8,9]. In 2006, Schnoes et al. [10] presented the results of a survey of sleep aid prescriptions for children by 222 pediatricians in 4 states, and clonidine was the second most prescribed sleep medication after antihistamines. Additionally, among 79 children who were prescribed clonidine for sleep disturbances, sleep onset difficulties (86.1%) were the most common indication and were substantially more common than other sleep problems (24.1%).
Clonidine is an imidazoline derivative that acts centrally on alpha-2 adrenergic receptors. Clonidine stimulates alpha-adrenoreceptors in the brainstem, which results in decreased sympathetic outflow from the central nervous system (CNS). This inhibition of sympathetic activity results in a decrease in peripheral resistance, renal vascular resistance, heart rate, and blood pressure, by which clonidine has been approved as an antihypertensive drug by the FDA. Clonidine also has other FDA-approved indications, such as ADHD in children, tics and cancer-related pain. Clonidine is used for a variety of “off-label” indications, including the treatment of anxiety, insomnia, and posttraumatic stress disorder, as well as the treatment of withdrawal symptoms from opioids, alcohol, and benzodiazepines. In particular, clonidine is also well known for being prescribed in children as a sleep medication. Although the exact mechanism has not been established, it has been hypothesized that clonidine induces somnolescence by decreasing norepinephrine (NE) release through the activation of α-adrenergic receptors in the CNS [8,11].
Although clonidine has been widely used as a sleeping medication in children and adolescents, there are only a few studies on how it affects sleep patterns in children and adolescents. While previous studies mostly focused on the association between clonidine and rapid eye movement (REM) sleep or the efficiency of clonidine as a sleep medication in children with or without ADHD, developmental delays, ASD, and genetic syndrome [8], the goal of this study was to investigate the effect of clonidine on sleep patterns in children and adolescents. Additionally, we examined whether clonidine has a better effect on patients with certain characteristics, such as age, sex, underlying psychiatric disorders, duration of clonidine use, etc. In addition, as sleep disorders are common comorbidities in patients with depression and anxiety, we attempted to confirm whether underlying depression or anxiety affects the effect of clonidine on sleep.
METHODS
Participants and procedures
This was a retrospective medical record review study conducted by the Catholic University of Korea, Eunpyeong St. Mary’s Hospital. A total of 71 children and adolescents aged 6 and 24 years who were prescribed clonidine as a sleep aid from the outpatient clinic of the Catholic University of Korea, Eunpyeong St. Mary’s Hospital between September 2019 and September 2021 were included in this study. The participants completed the Pittsburgh Sleep Quality Index (PSQI) before and 1 month after clonidine treatment for a retrospective chart review study. We reviewed the medical records of our participants to identify the principal diagnosis, concurrent medications, sleep complaints, and the response to clonidine treatment and to determine whether there were adverse effects after using clonidine. Their principal diagnoses were classified based on their diagnostic chart using International Classification of Diseases 10th Revision codes at the time of clonidine initiation for sleep purposes. The side effect profiles of the patients included in this study were recorded in the medical charts through the patients’ self-reports. This study was approved by the Institutional Review Board of Eunpyeong St. Mary’s Hospital, Seoul, Korea (IRB No. PC22RISI0009). Consent from individual subjects was not needed since it was a retrospective study.
Measures
Pittsburgh Sleep Quality Index
The PSQI is a self-report questionnaire that measures subjective sleep quality over the last 4 weeks with 18 items [12]. The first 4 items ask about times such as bedtime, the number of minutes it took for the participant to fall asleep, waking time, and hours of sleep per night. The next 10 items enquire how often the participant had trouble sleeping for different reasons [13]. It has 7 components, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. The score of each question is 0 (no difficulties) to 3 (serious difficulties), and the total score of the 7 components is from 0 to 21. The higher the score of the tool is, the worse the quality of sleep. Buysse et al. [12] classified the total score as indicating a “good sleeper” if the total score was below 5 points and “poor sleeper” if the total score was more than 5 points. Cronbach’s α for the scale was 0.788 in the current study. However, the Korean version of the PSQI (PSQI-K) developed by Sohn et al. [14] presented 8.5 points as a cutoff value. Cronbach’s α for the scale was 0.84 and 0.85 at the time of translation.
Children’s Depression Inventory
The Children’s Depression Inventory (CDI) is a self-report scale and is a modified version of Beck’s Depression Inventory that assesses depression in children and adolescents [15]. It contains 27 self-rated questions that reflect depressive symptoms, and participants are asked to select one of three alternative statements that best described their symptoms during the past 2 weeks. Each item earns either 0, 1, or 2 points, and higher scores indicate higher levels of depression [16]. For the present study, we used the Korean version of the CDI, which was developed by Cho and Lee [17], to measure levels of depression in children and adolescents. Scores using this scale were measured only at baseline in this study.
State-Trait Anxiety Inventory
The State-Trait Anxiety Inventory (STAI) is a self-report scale that measures the presence and severity of current symptoms of anxiety and a generalized propensity to be anxious with 40 items [18]. The State Anxiety Scale evaluates the current state of anxiety, including subjective feelings of tension, nervousness, worry, and arousal of the autonomic nervous system, by asking participants how they feel “right now.” It contains 20 items, with numerical values from 0 to 2. The Trait Anxiety Scale evaluates relatively stable aspects of anxiety proneness, such as general states of calmness, confidence, and security [19]. It contains 20 items, with numerical values from 0 to 2. Higher ratings indicate greater anxiety severity. Cronbach’s α for the scale was 0.853 in the current study. Scores using this scale were measured only at baseline in this study.
Statistics
Paired t tests were conducted to compare the sleep quality of the participants before and after clonidine treatment. Reliability analyses were performed to obtain Cronbach’s α for the questionnaires. Statistical significance for all tests was set at p<0.05. We conducted all statistical analyses using SPSS 21.0 for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
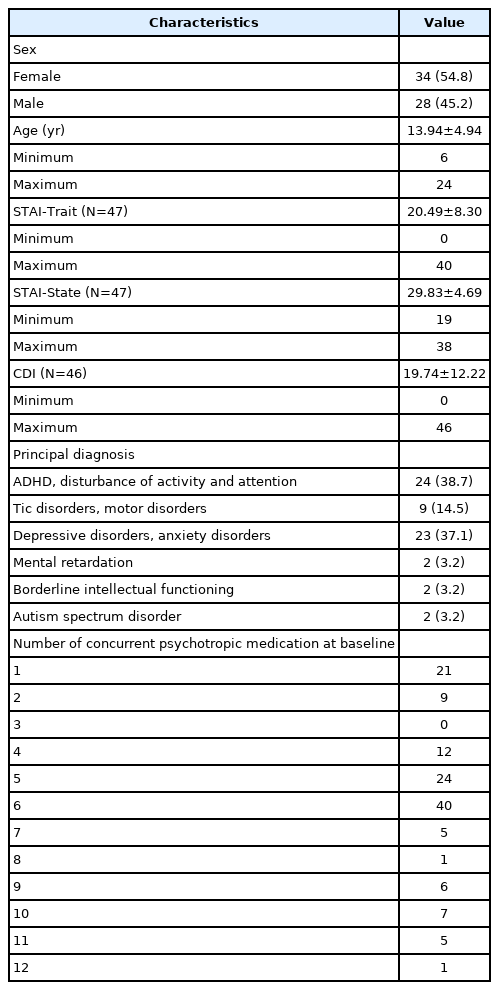
Of the 71 participants, 38 were female (53.5%), and the mean age was 13.48 years (standard deviation [SD]=4.88 years). Of them, 9 were excluded from the analysis because of incomplete responses, which resulted in a total of 62 participants for the final analysis. Of these participants, 34 were female (54.8%), and the mean age was 13.94 years (SD=4.94 years). In the group with ADHD and disturbance of activity and attention as the principal diagnosis, 2 participants had tic disorders, 11 had depression disorders, 3 had anxiety disorders, 1 had obsessive-compulsive disorder (OCD), 2 had mental retardation, and 1 had borderline intellectual functioning disorder, which were confirmed as comorbidities. In the group with tic disorders and motor disorders as the principal diagnosis, 6 participants had ADHD, 2 had depressive disorders, and 3 had anxiety disorders, which were confirmed as comorbidities. In the group with depressive disorders and anxiety disorders as the principal diagnosis, 3 participants had ADHD, 1 had OCD, and 2 had eating disorders, which were confirmed as comorbidities. In the group with mental retardation as the principal diagnosis, 1 participant had ADHD and 1 participant had a depressive disorder, which were confirmed as comorbidities. In the group with borderline intellectual functioning as the principal diagnosis, one participant had ADHD, which was confirmed as a comorbidity, and in the group with ASD as the principal diagnosis, two participants had ADHD, which was confirmed as a comorbidity.
Of the patients who used clonidine, 16 of 62 reported adverse side effects. Reported side effects included worsening of sleep disturbance (n=4), dizziness (n=4), dry mouth (n=3), oversedation (n=3), nausea (n=2), headache (n=2), irritability (n=1), numbness (n=1), skin rash (n=1), diplopia (n=1), tinnitus (n=1), lethargy (n=1), and general ache (n=1). The side effects were usually mild, and no serious side effects, such as angioedema, hypersensitivity, atrioventricular block, bradycardia, severe hypertension, or REM rebound, were found. Therefore, we concluded that the patients tolerated clonidine. The demographic and clinical characteristics of the patients in our study are presented in Table 1.
Association between clonidine use and Pittsburgh Sleep Quality Index component scores
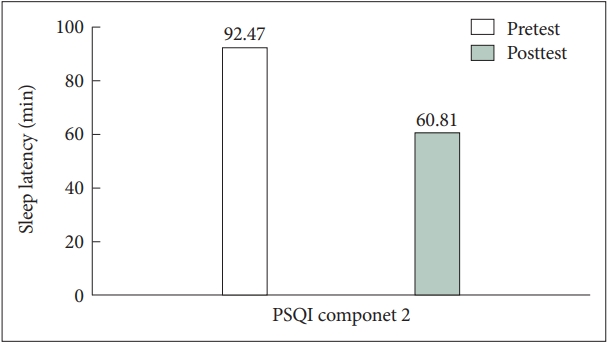
Most PSQI component scores decreased after clonidine administration, but only component 1, 2, and 5—subjective sleep quality, sleep latency, and sleep disturbance—were significantly reduced, as shown in Table 2. Specifically, we found that patients reported a more than 30 minutes shortening in mean scores of sleep latency time after using clonidine, as depicted in Figure 1 (mean±SD, 92.47±74.19 to 60.81±58.28 min). The mean scores of total sleep time increased by 12 minutes after using clonidine (mean±SD, 430.06±147.58 to 442.56±148.37 min). In addition, we discovered that component 6—use of sleeping medication—significantly increased, showing improvements in sleep quality and difficulties. By using clonidine, the patients reported that they could reach sleep more easily, sleep better, and be satisfied with their sleep.
Characteristics affecting the association between clonidine and sleep latency
The administration of clonidine significantly decreased the mean scores of component 2 of the PSQI in the female group but not in the male group (mean±SD; female: 2.38±0.74 to 1.79±0.91, p<0.001 vs. male: 2.46±0.79 to 1.96±0.96, p=0.017) (Table 3).
We divided the sample into two age groups: the 6–12-year-old group and the 13–24-year-old group. The criteria for classifying the two age groups were based on previous Centers for Disease Control and Prevention, American Academy of Sleep Medicine, and National Sleep Foundation recommendations, as well as commonly frequented websites [20]. The 13–24-year-old group showed significantly decreased mean scores of component 2 of PSQI compared with the 6–12-year-old group (mean±SD; 13–24 year group: 2.59±0.64 to 2.05±0.82, p=0.002 vs. 6–12 year group: 2.16±0.85 to 1.60±1.04, p=0.016) (Table 4).
Among the patients diagnosed with a variety of psychiatric disorders, patients with a principal diagnosis of ADHD or a mood disorder showed significant improvements in the mean scores of component 2 of the PSQI (mean±SD; ADHD: 2.29±0.75 to 1.63±0.77, p=0.003, mood disorders: 2.61±0.66 to 2.00±0.91, p=0.001) (Table 5).

Changes in sleep latency according to principal diagnosis at baseline and before and after treatment with clonidine
We divided the sleep latency of the participants at baseline into two groups: less than 60 minutes and more than 60 minutes. The more than 60 minute sleep latency group showed significantly decreased mean scores of component 2 of the PSQI compared with the less than 60 minute sleep latency group (mean±SD; above 60 min: 2.83±0.38 to 1.93±0.87, p<0.001 vs. less than 60 min: 2.03±0.82 to 1.81±1.00, p=0.229). We divided the duration of clonidine use into two groups: less than 14 days and more than 14 days. The group taking clonidine for more than 14 days showed significantly decreased mean scores on component 2 of the PSQI compared with the group taking clonidine for less than 14 days (mean±SD; more than 14 days: 2.47±0.77 to 1.78±1.02, p=0.001 vs. less than 14 days: 2.35±0.75 to 2.00±0.80, p=0.017) (Table 6).
Association between sleep improvement and underlying depression or anxiety after using clonidine
In this study, based on the cutoff value of 8.5 points on the total PSQI, the patients were divided into a good sleeper group (PSQI≥8.5) and a poor sleeper group (PSQI<8.5). Of the 62 patients, the number of good sleepers after using clonidine was 26, and the number of poor sleepers was 36. As shown in Table 7, the two groups showed no significant difference in diagnosis at baseline. However, the mean scores of STAI-Trait, STAI-State, and CDI of the poor sleeper group at baseline were higher than those of the good sleeper group, and only STAITrait and CDI mean scores were statistically significant (mean± SD; STAI-Trait of poor sleeper and good sleeper: 23.13±7.15 vs. 17.74±8.66, p=0.024, CDI of poor sleeper and good sleeper: 24.91±12.47 vs. 14.57±9.68, p=0.003) (Table 8).

Comparison of principal diagnosis at baseline between the good sleeper group and the poor sleeper group
DISCUSSION
Although clonidine has commonly been prescribed as a sleep medication for children and adolescents due to its sedative effects, neither well-randomized and controlled trials nor systematic studies of clonidine in sleep management exist [21]. To fill the gap in evidence, we investigated how clonidine affects the quality and quantity of sleep in children and adolescents by using a self-reported measure called the PSQI.
Interestingly, we found that most of the mean scores of PSQI components decreased after clonidine treatment, and specific components such as subjective sleep quality, sleep latency, and sleep disturbance significantly decreased. Specifically, clonidine was more effective in reducing sleep latency, showing more than 30 minutes shortening in mean scores of sleep latency. These results are consistent with those of previous studies showing that clonidine reduced sleep latency. Ming et al. [22] showed that clonidine was effective in reducing sleep latency and night awakening in children with ASD. Ingrassia and Turk [23] described the benefit of clonidine on sleep initiation, night-time wakening and early morning awakening in 6 children with intellectual disabilities. These findings suggest that clonidine is efficacious in managing sleep disturbances, especially in sleep latency disorders of children and adolescents, by improving the quality and quantity of sleep.
In addition, we examined whether the effectiveness of clonidine on sleep latency differs depending on certain factors, including sex, age, psychiatric comorbidities, and sleep latency time at baseline. Compared to the other groups, it was particularly significant that the sleep latency was more shortened in the female group, 13–24-year-old group, group with a principal diagnosis of ADHD or a mood disorder, and group with a sleep latency of more than 60 minutes at baseline.
Both the male and female groups exhibited a reduction in sleep latency after clonidine treatment, but the difference was more pronounced in the female group. In a previous study comparing sex differences in catecholamine secretion after clonidine, NE plasma levels were significantly decreased more in women than in men. This finding supports the results of our study that clonidine treatment shows sexual dimorphism when used as a sleep aid, regarding the most likely hypothesis that clonidine induces sleep by decreasing NE secretion with agonism of α 2-adrenergic receptors [24].
In this study, sleep latency was more significantly reduced in the 13–24-year-old group than in the 6–12-year-old group. Individually, the sleep habits of children undergo changes through childhood and adolescence, and aging may lead to a late bedtime and short sleep duration. Furthermore, specific sleep-related difficulty areas may correspond to specific age groups. For instance, bedtime resistance and difficulty sleeping through the night are often reported in younger people, while difficulty falling asleep and daytime sleepiness become more common in older people [2,25]. Usually, it is known that adolescents are accompanied by a delay in nocturnal melatonin secretion and altered “sleep impulse,” in which the pressure to fall asleep accumulates more slowly than in adults. In addition to the sleep-wake changes from this biological maturation, adolescents undergo increased academic demands and occasionally deal with part-time jobs, extracurricular and social activities and a high use of electronic devices [26-28]. For the preceding reasons, problems in sleep latency at baseline are more likely to occur in the 13–24-year-old group than in the 6–12-year-old group, which was found in this study (M±SD; 13–24-year-old, 2.59±0.64 vs. 2.16±0.85) (Table 4). Although the clear mechanism by which clonidine reduces sleep latency has not yet been elucidated, several studies, including this study, have shown that clonidine is significantly efficacious in reducing sleep latency, suggesting that clonidine may be more effective in the 13–24-year-old group, who suffer more from subjective difficulty in sleep onset than the 6–12-year-old group. To prove that there is a difference in the effect of clonidine according to age, further studies on the mechanism by which clonidine affects sleep latency should be prioritized.
For the patients with a principal diagnosis of ADHD or a mood disorder, the study showed a significant reduction in sleep latency after using clonidine. The mean scores of PSQI component 2 decreased from 2.29 (SD=0.75) to 1.63 (SD=0.77) in the ADHD group and from 2.61 (SD=0.66) to 2.00 (SD=0.91) in the mood disorder group. According to parents’ reports, 25% to 50% of children and adolescents with ADHD are known to have sleep disturbances [29]. Another study reported that 73.3% of 239 children diagnosed with ADHD had mild to severe sleep disorders [30]. Sleep disorders commonly reported by children and parents include difficulties initiating sleep, maintaining sleep, tiredness upon waking and daytime sleepiness [30,31]. It has been hypothesized that the sleep problems seen in ADHD children or adolescents are associated with either adverse effects of stimulant usage, core ADHD symptoms, a psychiatric comorbidity, or a combination of them [9]. Despite differences based on the type, dose, and duration of drug sue, it has been reported that the sleep onset latency is longer than 30 minutes when using stimulants [32]. Additionally, ADHD has a variety of comorbid psychiatric diseases, and comorbidities such as mood disorders, anxiety disorders, and tic disorders have all been associated with significant sleep disturbances [33,34]. Our study showed a significant sleep latency reduction in children and adolescents diagnosed with ADHD or diagnosed with mood disorders/anxiety disorders, which are commonly comorbid with ADHD. This is consistent with the results of previous studies. In a more recent study, more than 85% of children and adolescents with anxiety disorders reported sleep disturbances, and the most common sleep disorders in children with anxiety disorders are sleep initiation and maintenance difficulties, frequent nocturnal awakening, bedtime refusal, co sleeping, nightmares, and nocturnal fear [34,35]. Forbes et al. [36] conducted a large, controlled study using both subjective sleep reports and polysomnography (PSG) in children and adolescents with anxiety disorder and major depression. Adolescents with anxiety disorders showed more sleep disruption on the PSG with an increased number of awakenings and fewer slow waves and had longer sleep latency than children with major depression and controls [34]. In an initial study of prepubertal children diagnosed with major depressive disorder, approximately 66% reported sleep onset difficulties and sleep maintenance problems. Early studies identified considerably shorter REM latency and greater REM density in depressed adolescents than in controls [34,37]. Although sleep disturbance is often comorbid with depression, sleep disturbance is also known to be a common risk factor for subsequent clinical depression [38]. According to the above study results, patients diagnosed with anxiety disorder showed lower slow wave sleep, whereas patients diagnosed with depressive disorder showed shortened REM latency and increased REM density. Because clonidine may affect REM latency and slow wave sleep, it could be inferred to be a reason clonidine shows more improvement in sleep latency in patients diagnosed with mood disorders/anxiety disorders.
Furthermore, we found that the mean scores of PSQI component 2 decreased from 2.35 (SD=0.75) to 2.00 (SD=0.80) in patients who took clonidine for less than 2 weeks, whereas the mean scores of PSQI component 2 decreased significantly from 2.47 (SD=0.77) to 1.78 (SD=1.02) in patients who took clonidine for more than 2 weeks. Because clonidine is known to produce dose-related sedation, it may be that more improvement was shown in sleep latency after using clonidine for more than 2 weeks than when using it for less than 2 weeks [11,39].
Moreover, this study found that poor sleepers, who had total scores above 8.5 on the PSQI after clonidine administration, had higher scores on the STAI-Trait and CDI at baseline than good sleepers, who had total scores below 8.5 on the PSQI. Contrary to the results of this study, which showed a significant decrease in PSQI component 2 after using clonidine in the group with comorbid mood disorder/anxiety disorder, it was confirmed that the total PSQI score after using clonidine was higher as the underlying depression and anxiety increased. This result might be explained by the fact that the mood disorder/ anxiety disorder group already had high total scores, not only for the baseline PSQI component but also for other PSQI components. However, the STAI and CDI scores after using clonidine were not evaluated in this study. Therefore, our results could hardly jump to the conclusion that the levels of depression and anxiety are negatively correlated with the effectiveness of clonidine on sleep. Considering this, we recommend future studies to use a scale that has objective evaluations of experts along with STAI and CDI. This might support our results that levels of depression and anxiety at baseline could affect the effectiveness of clonidine in sleep.
Currently, there is no FDA-approved medication for sleep disorders in children and adolescents, but clinically, various medications are being used. Antihistamine—especially diphenhydramine and hydroxyzine—are the most frequently prescribed drugs for insomnia alone, followed by α2-adrenergic agonists, melatonin, tricyclic antidepressant, and other antidepressants [10]. Although antihistamines are known to be the most prescribed agents for insomnia, there are not enough randomized controlled studies in the pediatric population. Their sedative effects are due to its lipid solubility and resultant ability to penetrate the blood–brain barrier to block the H1-receptor. It is also known to reduce sleep latency and increase sleep continuity [10,40-43]. However, as an antihistamine is also a multifunctional drug that blocks muscarinic cholinergic and adrenergic receptors, it may lead to fever, mydriasis, blurred vision, dry mouth, urinary retention, constipation, tachycardia, dystonia, and confusion as side effects. Five cases of fatal diphenhydramine toxicity have also been reported in case series for newborns at 6–12 weeks [42,44,45]. Another of the most prescribed sleep medications, melatonin, activates MT1 and MT receptors by modulating the sleep/wake cycle. Additionally, previous studies have found that melatonin is efficacious in enhancing total sleep time, sleep latency, and number of waking incidents [46,47]. According to a recent meta-analysis, 9 previous studies found that melatonin enhanced total sleep time compared to placebo (mean difference [MD]=48.26 min; 95% confidence interval [CI], 36.78 to 59.73). Similarly, sleep latency was reduced in the groups that used melatonin in 11 previous studies (MD=-28.97; 95% CI, -39.78 to -18.17). Although drug-related serious adverse events were not reported, there was no difference in nocturnal awakenings after using melatonin (MD=-0.49; 95% CI, -1.71 to 0.73) [48].
A clear primary mechanism of how clonidine produces sleep has not been identified, and the most widely known hypothesis is that clonidine produces somnolence by decreasing NE via negative feedback by agonism of the alpha 2-adrenergic receptors, which would increase REM sleep [49]. However, several studies, including this study, have confirmed that clonidine is effective in reducing sleep latency. The fact that clonidine takes within 1 to 3 hours to reach peak plasma concentration by oral dosing may explain the significant reduction in sleep latency after using clonidine. Additionally, clonidine has the advantage of being used as an adjuvant therapy for children and adolescents not only with sleep disorders but also with ADHD, behavior problems, hypertension, Tourette disorder, tic disorder, and ASD. However, since clonidine was originally approved as an antihypertensive drug, some side effects have been reported after its use. The most common side effects included headache, abdominal pain, nausea and fatigue, and in relation to anticholinergic effects, they also included constipation, sexual dysfunction and xerostomia. Additionally, serious side effects included angioedema, hypersensitivity, atrioventricular block, bradycardia and severe hypotension. Furthermore, because of REM suppression action, rapid discontinuation may be accompanied by hypertension and REM rebound, so caution is required when using it [11,42]. A study of 1,060 children under the age of 19 reported that compared to that in 1993, the use of clonidine medication in 1999 increased from 2.6% to 13.1% under the age of 6, from 22.1% to 40.4% between the ages of 6 and 12, and from 16.7% to 25.5% between the ages of 13 and 18. As the use of clonidine increases, there is some evidence that the trend of overdose of clonidine may be on the rise. However, a small number of clonidine overdoses result in fatality. Of these, 78.7% of exposures showed no effect or minor effect, and only 1 fatality was reported. In addition, the side effects of clonidine subside or decrease over time and are dose dependent [8,50,51]. Similarly, although our study was preliminary and retrospective, we found that 50 of 62 patients (80.6%) using clonidine were well-treated without even mild side effects. Although clonidine has not shown better effects or higher safety than other common sleeping drugs (i.e., antihistamine, melatonin) in children and adolescents, there are no FDA-approved sleeping drugs for children and adolescents at present. Therefore, it is important to note that using clonidine could be a more appropriate approach, as there are no sleeping drugs that show superiority over other drugs.
This study should be considered in the context of its strengths and limitations. Most importantly, we highlight the effectiveness of clonidine as a sleep medication for children and adolescents. In addition to the findings that clonidine may improve the quality and quantity of sleep in children and adolescents, our study suggests that the patient’s specific characteristics at baseline may impact the effectiveness of clonidine on sleep latency. This study will be useful as basic data for future research exploring the mechanism by which clonidine produces sleep. Additionally, by confirming its clinical effect, it can be expected that clonidine can be more actively used to treat insomnia and improve sleep quality in children and adolescents. However, our study has several limitations. First, as we conducted a retrospective study, we used the medical charts of our sample, which may have resulted in the possibility of interpreting our study results with expectancy bias. Second, this study had a limitation in that it did not correct for the confounding effect of the use of concurrent psychotropic drugs that may affect sleep while using clonidine. Third, this study focused on self-reported measures of sleep, and objective measures such as PSG or actigraphy were not available. Fourth, the relatively small sample size and the lack of a control group limited the power to demonstrate the effects of clonidine. Fifth, this study was conducted by an institution in South Korea, so it is difficult to generalize the results across the country or to other races. Sixth, because this study was conducted over a short period of time, further long-term studies on clonidine use and sleep latency should be performed to understand the durability of the clinical results of this study. In addition, this study broadly divided the participants into two groups: children aged 6–12 years and adolescents aged 13–24 years. In children and adolescents, changes in sleep architecture may occur according to developmental changes. Therefore, to compare the effects of clonidine on sleep latency according to age, we suggest that further research including a more subdivided comparison of age groups according to the developmental stage of children and adolescents is needed. Last, considering that the aforementioned side effects might occur in a dose-dependent manner, we recommend that future studies establish safety when clonidine is used in children and adolescents for long-term sleep purposes.
In conclusion, this study examined the effectiveness of clonidine as a sleep medication for children and adolescents through the PSQI of 62 children and adolescents with sleep difficulties. We established that clonidine significantly improved subjective sleep quality, sleep latency and sleep disturbance after treatment. It is particularly significant that the female group, 13–24-year-old group, group with a principal diagnosis of ADHD or a mood disorder, and group with a sleep latency time of more than 60 minutes at baseline reported a greater reduction in their sleep latency according to component 2 of the PSQI. In addition, clonidine was verified to be more effective when consistently administered for more than 2 weeks. However, the higher the levels of depressive symptoms and anxious symptoms at baseline were, the lower the improvement in sleep after using clonidine. These findings suggest that clonidine is a viable option for clinicians treating children and adolescents with sleep disorders, while there are no FDA-approved medications for insomnia in the child and adolescent population.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Min-Hyeon Park, a contributing editor of the Psychiatry Investigation, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Min-Hyeon Park. Data curation: Jae Yeon Woo, TaeHyeong Kim, Min-Hyeon Park. Formal analysis: Haemi Choi, Jae Yeon Woo. Investigation: Haemi Choi, Tae-Hyeong Kim. Methodology: Dajung Sung, Min-Hyeon Park. Project administration: Min-Hyeon Park. Resource: Min-Hyeon Park. Software: Haemi Choi, Jae Yeon Woo. Supervision: Min-Hyeon Park. Validation: Young-Jin Jang, Tae Sun Han, Min-Hyeon Park. Visualization: Haemi Choi, Jae Yeon Woo. Writing—original draft: Young-Jin Jang. Writing—review & editing: Haemi Choi, Tae Sun Han, Dajung Sung, Min-Hyeon Park.
Funding Statement
None