Valproate Adjuvant Cognitive Behavioral Therapy in Panic Disorder Patients With Comorbid Bipolar Disorder: Case Series and Review of the Literature
Article information
Abstract
Anxiety disorders are the most common comorbid psychiatric disorders in patients with bipolar disorder. Managing anxiety symptoms in comorbid conditions is challenging and has received little research interest. The findings from preclinical research on fear conditioning, an animal model of anxiety disorder, have suggested that memory reconsolidation updating (exposure-based therapy) combined with valproate might facilitate the amelioration of fear memories. Here, three cases of successful amelioration of agoraphobia and panic symptoms through valproate adjuvant therapy for cognitive behavioral therapy in patients who failed to respond to two to three consecutive standard pharmacotherapy trials over several years are described. To the best of the author’s knowledge, this is the first attempt to combine CBT with valproate in patients with panic disorder, agoraphobia, and comorbid bipolar disorder. Additionally, the background preclinical research on this combination therapy based on the reconsolidation-updating mechanism, the inhibition of histone deacetylase 2, and critical period reopening, off-label use of valproate in panic disorder, plasticity-augmented psychotherapy, and how to combine valproate with CBT is discussed.
INTRODUCTION
Bipolar disorder has a high comorbidity rate (40%) with anxiety disorders [1]. Approximately 15% of people with bipolar disorder are diagnosed with panic disorder during their lifetime [2]. The comorbidity of anxiety disorders, especially panic disorder, negatively affects the clinical course of bipolar disorder and is correlated with poor treatment outcomes [3], a greater risk of suicide [4], substance misuse [5], and an earlier age of bipolar disorder onset [1,6].
Panic disorder seems to share a genetic susceptibility with bipolar disorder. Patients with bipolar disorder are highly sensitive to anxiety [7], panic disorder during adolescence is a risk factor for developing bipolar disorder in adulthood [8], and there is a higher prevalence of panic disorder in first-degree relatives of patients with bipolar disorder than in those of patients with unipolar depression and recurrent depression [9,10]. Structural and functional neuroimaging studies have revealed a shared pathology between bipolar disorder and panic disorder; for example, the reactivity of a small area of the amygdala [11] increases/decreases during a manic/depressive episode in bipolar disorder [12]. A gray matter volume reduction in the amygdala [13,14] and its hyperactivity in response to stimuli [15] have also been reported in panic disorder. The amygdala and ventral prefrontal cortex-amygdala network play a key role in mood regulation in bipolar disorder [16] and the regulation of fear and anxiety [17].
However, studies on treatment for comorbid panic disorder and bipolar disorder are scarce, and treatment guidelines are based on a small number of studies with low levels of evidence [18]. The lack of effective interventions to manage anxiety symptoms may lead clinicians to prescribe more benzodiazepines or patients to self-medicate more (e.g., with alcohol) [19].
The treatment of panic symptoms in patients with comorbid bipolar disorder is challenging. Antidepressant treatment, the first-line treatment for panic disorder, is controversial [20,21], as it can lead to mood destabilization in patients with bipolar disorder [22]. Bipolar disorder patients with panic disorder who take antidepressants have a greater risk of manic switching [20,21]. The risk of manic switching is especially high in patients showing panic symptoms concomitantly during manic episodes [21]. The Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines recommend quetiapine and gabapentin as first-line treatments for anxiety disorders in patients with bipolar disorder [18,23]. Valproate, lamotrigine, serotonergic antidepressants, and olanzapine are second-line treatments [18,23]. Cognitive behavioral therapy (CBT) has been recommended as the first-line treatment for anxiety management in adolescents [18,23]. However, none of the above treatment options have been shown to be better than others in randomized controlled clinical trials. Although the treatment of panic disorder comorbid with bipolar disorder is clinically important, associated research is lacking.
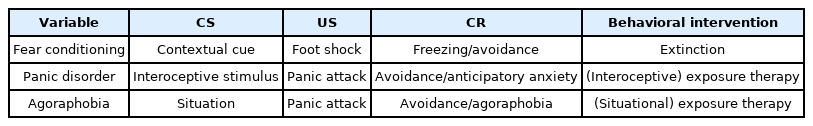
CBT for panic disorder, developed by Barlow [24], demonstrates efficacy and is regarded as a first-line treatment for this disorder [25]. An interoceptive fear conditioning model of panic disorder [24,26,27] explained that susceptible people (i.e., those with biological vulnerability or stress due to negative life events) experience panic attacks (unconditioned stimulus [US]) incidentally and then later associated with the interoceptive cues (conditioned stimulus [CS]; e.g., physical sensations such as palpitations, shortness of breath, and dizziness) (Table 1). Agoraphobia can also be explained through fear conditioning. Repetitive panic attacks (US) are associated with the contextual situation (CS) in which an attack is experienced. Affected individuals then generalize this association to similar situations, avoid causal situations, and restrict everyday life activities (agoraphobia). In exposure therapy, patients face situations that induce agoraphobia without avoiding them. Exposure therapy with interoceptive and situational exposure is an active component of CBT for panic disorder (Table 1) [24]. Recently, attempts have been made in the field of posttraumatic stress disorder (PTSD) to increase the effectiveness of CBT with the help of drugs. Patients with PTSD showed a better response to exposure-based treatment when propranolol was used immediately before the retrieval of traumatic memories [28].
Based on the similarities in the pathogeneses of panic disorder, agoraphobia and PTSD, it may be possible to improve CBT through the study of rodent models, such as fear conditioning and extinction training (a rodent analog of exposure therapy). The successful use of antidepressants [29] or propranolol [30] combined with exposure-based therapy in rodent models has been reproduced in clinical studies of anxiety disorders [24,31,32] as well as PTSD [28]. Based on these successful translational studies, the fear conditioning model has high predictive validity in relation to anxiety disorders and is a good model for discovering new therapeutic interventions.
Anecdotal evidence suggests that valproate may be an alternative treatment for panic disorder [33-36], antidepressant-resistant panic disorder [37,38], and panic disorder with comorbid bipolar disorder [33]. However, these initial findings have not been substantiated by subsequent studies. Randomized controlled studies comparing each treatment option with placebos are lacking. Therefore, in clinical guidelines for panic disorder, valproate remains a tertiary adjuvant option with little evidence [39,40] or it is not mentioned [41].
Valproate has anxiolytic effects through its promotion of gamma-aminobutyric acid [42] and acts as a histone deacetylase 2 (HDAC2) inhibitor. HDAC2 inhibition has been shown to improve learning and memory by enhancing neural plasticity [43]. Extinction training in combination with valproate facilitates the attenuation of learned fear in rodent models through effective inhibition of HDAC2 by valproate [44,45]. Moreover, an HDAC2 inhibitor (CI-994) successfully inhibited treatmentresistant remote fear memories in mice [46]. Except for valproate, HDAC2 inhibitors (e.g., CI-994) cannot be applied in the treatment of anxiety disorders, as they have received Food and Drug Administration (FDA) approval only for chemotherapy [47]. Thus, inspired by the synergistic effects of HDAC2 inhibitors and exposure-based therapy in a rodent model of anxiety disorders, the author hypothesized that valproate, a clinically available HDAC2 inhibitor, might enhance the treatment response to CBT in patients with comorbid panic disorder and bipolar disorder.
Here, the cases of three patients with comorbid panic disorder and bipolar disorder treated with valproate adjuvant therapy for CBT are presented. Additionally, the background preclinical research on this combination therapy based on the reconsolidation-updating mechanism, the inhibition of HDAC2, critical period reopening, off-label use of valproate in panic disorder, plasticity-augmented psychotherapy, and how to combine valproate with CBT is discussed.
CASE SERIES
Case 1: bipolar I disorder, panic disorder, agoraphobia
A woman in her 30s visited our outpatient clinic. At 25 years of age, she experienced her first major depressive episode. After taking antidepressant medications, her mood shifted to a manic episode. While taking medication (lithium 750 mg, lamotrigine 75 mg, and quetiapine 100 mg daily), she attempted suicide twice, including slashing her wrists and attempting to overdose on drugs. The patient stopped taking the medication after experiencing lithium intoxication. She quit her job after the onset of mood symptoms and suffered from panic disorder and agoraphobia. She could not enter crowded cafés or supermarkets because of the fear that others would see her panic attacks. On her first visit, valproate 750 mg, quetiapine 100 mg, and clonazepam 0.25 mg were prescribed because she wanted to take a mood stabilizer other than lithium. To relieve her agoraphobia and regain occupational functionality, she underwent CBT. During the 12 sessions, the patient took the medications mentioned above. The relaxation technique and systematic desensitization to agoraphobia-inducing situations gradually relieved her avoidance of causal activities. Her initial Panic Disorder Severity Scale (PDSS) score was 17; however, after 12 sessions, it decreased to 0 (Figure 1). She no longer took a taxi instead of public transportation to go to the hospital, and she could go out for coffee or enter crowded buildings by herself. She attended a job interview and began to work again. Agoraphobia and panic symptoms had not recurred at the six-month follow-up after CBT completion.

Improvement in panic symptoms in patients with panic disorder and comorbid bipolar disorder after valproate adjuvant treatment for CBT. Three patients with panic disorder and comorbid bipolar disorder were treated with valproate in combination with CBT. Before the start of each session, symptom severity was assessed using the PDSS (session 1: pre-treatment; session 13 or 16: post-treatment). Valproate was used as maintenance treatment for bipolar disorder (new: case 1; ongoing: case 2) or as adjuvant treatment for CBT (case 3). The patients showed an improvement in panic symptoms after 12–15 sessions of CBT. The effect was maintained for at least six months after CBT was terminated. PDSS, Panic Disorder Symptom Scale; CBT, cognitive behavioral therapy.
Case 2: bipolar I disorder, panic disorder, generalized anxiety disorder
A woman in her 40s visited our outpatient clinic complaining of recurrent panic attacks. She had a family history of bipolar disorder and experienced postpartum depression with psychotic features at the age of 30. She was diagnosed with bipolar I disorder and prescribed escitalopram 10 mg, alprazolam 1.5 mg, quetiapine 25 mg, and valproate 300 mg, which she took for three years. She was diagnosed with generalized anxiety disorder and panic disorder according to Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) criteria. Her anxiety symptoms were not alleviated after three years of taking the above medications. She was worried that she might attempt suicide. The patient also exhibited hypochondriasis and somatic symptoms. After careful evaluation, the patient was prescribed a new medication, escitalopram (valproate 500 mg, quetiapine 100 mg, and alprazolam 1.5 mg). She underwent 15 sessions of CBT targeting her panic disorder and generalized anxiety disorder. Compared with the standard 12 sessions of CBT for panic disorder, to teach her how to cope with excessive worry, the proportion of cognitive reappraisal techniques was increased, and the treatment was extended to 15 sessions. Her initial PDSS score was 14, which decreased to 7 after 15 sessions of CBT. At the six-month follow-up, she had symptoms of generalized anxiety but no panic attacks (Figure 1).
Case 3: bipolar II disorder, panic disorder, agoraphobia
A woman in her late 20s visited our outpatient clinic. She had experienced a major depressive episode at 14 years of age. She was diagnosed with panic disorder and agoraphobia in her 20s and had a history of inpatient treatment for major depressive episodes with psychotic features. She failed to maintain her academic and occupational functioning because of depressive symptoms and agoraphobia. Although she had been taking antidepressant medications, including a selective serotonin reuptake inhibitor (SSRI) and norepinephrine dopamine reuptake inhibitor (fluoxetine 20 mg, bupropion SR 150 mg), for more than 5 years, her depressive symptoms had not diminished, and she had attempted suicide. The patient experienced hypomanic episodes during the follow-up period. The patient was diagnosed with bipolar II disorder. Her medication was changed to quetiapine (400 mg) and lithium (750 mg). However, she still experienced recurrent panic attacks, had trouble using public transportation, and was reluctant to visit crowded places such as supermarkets. Thus, she decided to undergo CBT and completed 12 sessions. Valproate 750 mg was added to her medication regimen during the final five sessions, which encompassed exposure training in agoraphobia-provoking situations. The patient’s PDSS score decreased to 1 from an initial score of 11. After the completion of CBT, valproate was reduced, and her agoraphobia was successfully alleviated. At the 6-month follow-up, her panic disorder and agoraphobia symptoms remained in remission (Figure 1).
DISCUSSION
In these three cases, the combination of valproate and CBT in patients with bipolar disorder who did not respond to previous standard treatments successfully managed panic symptoms and agoraphobia. Agoraphobia, which can be diagnosed alone but is typically accompanied by panic disorder, is a clinical condition associated with poor prognosis in the treatment of panic disorder [48].
Comorbid bipolar disorder is also regarded as a poor prognostic factor for the treatment of panic disorder [49]. In the three cases above, two patients (cases 1 and 3) had suffered panic disorder with agoraphobia for a long period of time, and none of the patients responded to two or three consecutive trials of pharmacotherapy, including quetiapine combined with valproate (cases 1 and 2) and quetiapine combined with lithium (case 3). In case 2, valproate was used previously, and in case 1, valproate was started when CBT was started; valproate continued to be used in both cases even after CBT was terminated. However, in case 3, because lithium and quetiapine were already being used for maintenance treatment, valproate 750 mg was used only temporarily during CBT; nonetheless, the effect was maintained without the recurrence of agoraphobia or panic disorder for six months after the treatment (Figure 1).
Here, the author reports a case series of successful treatment of panic symptoms and agoraphobia in patients with comorbid bipolar disorder through valproate adjuvant treatment for CBT (Figure 2). However, a few limitations of this study should be mentioned. Mood symptoms, often accompanied by panic symptoms, were not objectively monitored throughout the CBT course in the case series. The serum concentrations of valproate were not determined over the course of treatment. The author previously published a review article that comprehensively explained in detail the theoretical background of combining valproate and CBT [50]. Here, the theoretical background of valproate and CBT is briefly described in the following sections (Figure 2).
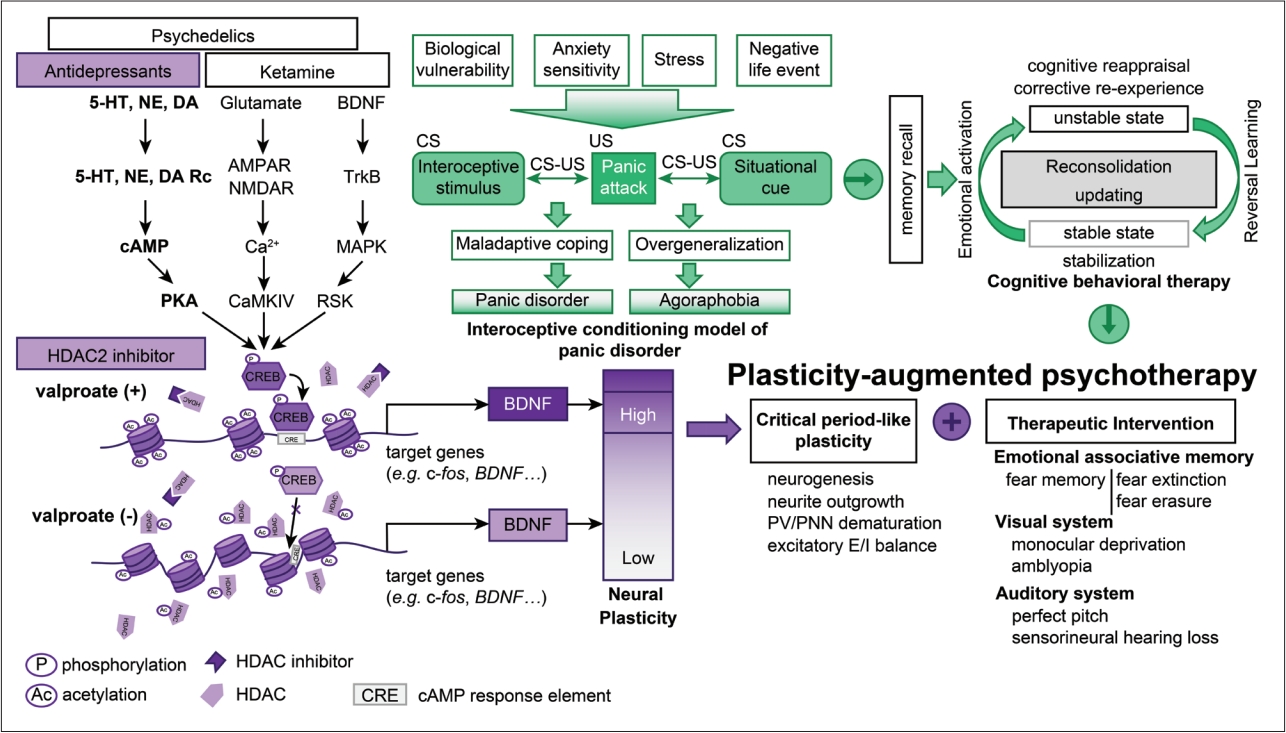
Schematic diagram of valproate adjuvant treatment for CBT. Plasticity-augmented psychotherapy is a framework for understanding the mechanism of action in combined pharmacotherapy and CBT. Activity-dependent neural plasticity is the foundation for adaptation to the environment. Antidepressants, ketamine, and psychedelics, such as MDMA and HDAC2 inhibitors, enhance neural plasticity by modulating neurotransmitters and epigenetic modifications, such as histone acetylation. Critical period-like plasticity not only offers an opportunity to repair developmental impairments in sensory processing but also allows the correction of dysfunctional behavioral symptoms. The reconsolidation-updating paradigm activates a memory engram that supports maladaptive coping behavior in panic disorder and agoraphobia. Consequently, the memory engram becomes malleable to the environment within a limited time window. Therapeutic interventions such as CBT should be provided to patients during this period. CBT, cognitive behavioral therapy; PNN, perineuronal net; PV, parvalbumin interneurons; E/I, excitatory/inhibitory; 5-HT, 5-hydroxytryptamine; NE, norepinephrine; DA, dopamine; BDNF, brain-derived neurotrophic factor; Rc, receptor; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; NMDAR, N-methyl-D-aspartate receptor; TrkB, tropomyosin receptor kinase B; cAMP, 3′,5′-cyclic adenosine monophosphate; MAPK, mitogen-activated protein kinase; PKA, protein kinase A; CaMKIV, calcium/calmodulin-dependent protein kinase type IV; RSK, ribosomal s6 kinase; CREB, cAMP response element-binding protein; CRE, cAMP response element; HDAC, histone deacetylase; MDMA, 3,4-methylenedioxy-methamphetamine; US, unconditioned stimulus; CS, conditioned stimulus.
Reconsolidation updating and psychological treatment
Memory reconsolidation is a phenomenon observed in various categories of memory, including emotional memory, in both animals and humans and explains the behavioral changes that occur during psychotherapy [50]. The memory is strengthened, weakened, or updated in response to environmental stimuli immediately after memory recall, a process known as memory reconsolidation. The counterpart of memory reconsolidation is memory consolidation immediately following memory acquisition. Memory consolidation and reconsolidation involve shared and distinct cellular, molecular, and synaptic processes [51,52].
The amygdala is essential for the formation and recall of emotional memories associated with positive and negative valence emotions, such as fear memory [53,54]. The formation and recall of these emotional memories occur through unconscious processes. Transference and “working through” in psychoanalysis are good examples of the reconsolidation of emotional memories.
It is difficult to eradicate a fear memory once it is formed. Extinction training, which repetitively presents the CS without US, can only transiently inhibit the expression of fear memory. After time has elapsed, the learned fear re-emerges (spontaneous recovery) [55]. However, very young mice are highly resilient in terms of recovery after extinction training, without spontaneous recovery (Figure 3) [56]. Chronic antidepressant treatment can rejuvenate neurons in adult mice to eliminate fear memories without causing relapse (Figure 3) [29].
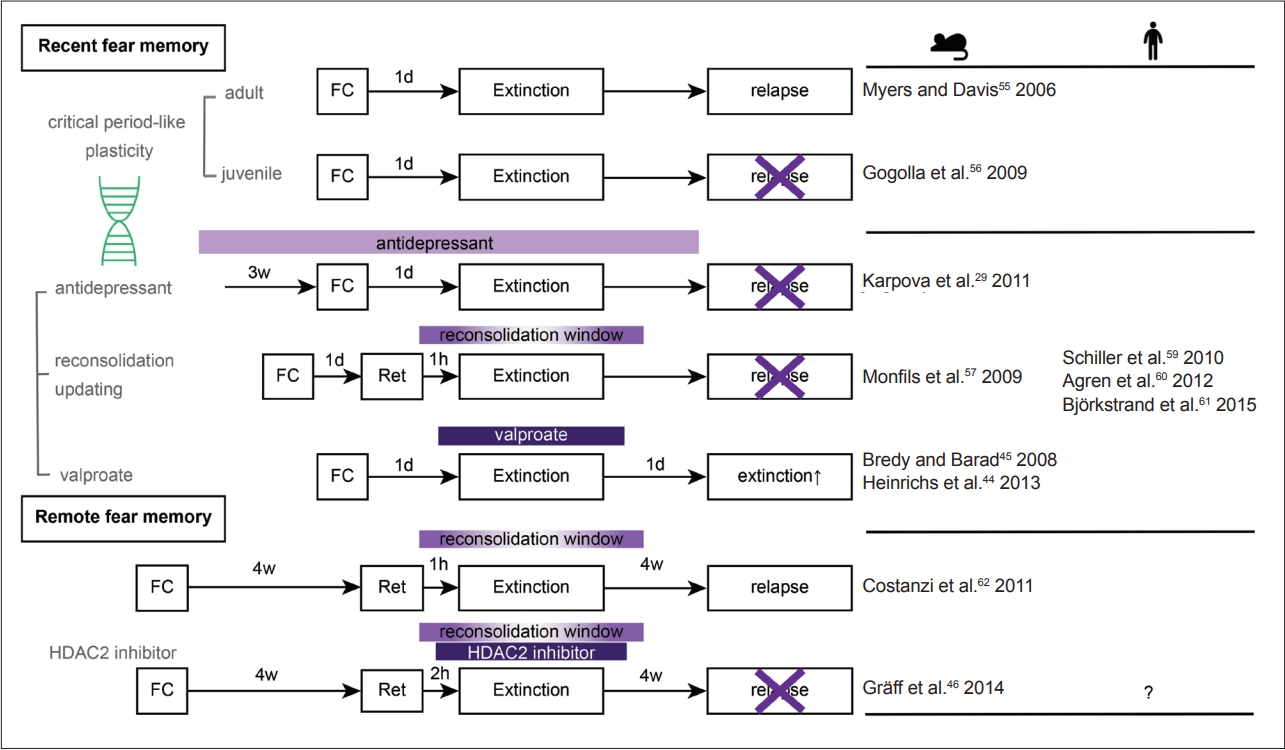
Preventing the return of fear memories with pharmacological and behavioral interventions. Fear memories are reluctant to be persistently suppressed by extinction. Unlike adult mice, juvenile mice exhibit enhanced neural plasticity to such an extent that their fear memories are erased by extinction. This juvenile-like plasticity can be achieved by various interventions, such as chronic antidepressant treatment, reconsolidation updating, and HDAC2 inhibition. Remote fear memories, which are more difficult to persistently suppress by extinction, can be successfully suppressed by a combination of reconsolidation updating and HDAC2 inhibition. Valproate, a clinically available HDAC2 inhibitor, is known to facilitate the extinction of recent fear memories. Ret, retention (retrieval); FC, fear conditioning; d, day; w, week; h, hour; HDAC, histone deacetylase.
With no pharmacological intervention, by presenting CS 1 hour before extinction training, a recent fear memory can be successfully erased without spontaneous recovery [57]. During the reconsolidation window (10 minutes–6 hours), neurons that participate in the memory engram are activated and malleable in response to environmental stimuli [58]. Adequate learning during the reconsolidation period profoundly rewrites the preexisting emotion-associated memory, a process termed “reconsolidation updating.”
This reconsolidation update has been proven to work well in humans when applied to recent fear memories (Figure 3) [59-61]. However, as in animal experiments, the application to remote fear memories (1 month) was not successful in consistently reducing the fear response [62]. The reconsolidation-updating phenomenon is regulated by brain-derived neurotrophic factor (BDNF). BDNF Met variants, which are reported to be associated with low BDNF expression and thus less neuroplasticity, were associated with a non-persistence of these effects [63].
This reconsolidation process is an adaptation strategy that allows individuals to modify already-formed behaviors when circumstances change [58]. Memories should be both persistent and rewritable and should be regulated by coordinated mechanisms at the synaptic, cellular, and transcription levels [64]. Epigenetic modifications, such as histone acetylation and deacetylation, regulate the transcription of target proteins involved in the consolidation and reconsolidation of memory. Increased histone acetylation by histone acetyl transferase in the target gene promoter increases gene expression, whereas decreased histone acetylation through increased HDAC activity decreases the transcription of target genes (Figure 2).
Remote fear memories are less able to be modified after memory recollection than recent fear memories (Figure 3) [62]. The histone acetylation level after the recall of remote fear memories is significantly less than that after the recall of recent fear memories. Histone acetylation is upregulated by histone acetylases and antagonistically downregulated by histone deacetylases. Pharmacologically, HDAC2 inhibition (by CI-994) has been shown to restore histone acetylation activity to the level of that with recent fear memory recall after memory recall during the reconsolidation window and extinction training and to successfully erase remote fear memories without later relapse (Figure 3) [46].
Valproate as an histone deacetylase 2 inhibitor and critical period reopening
The cellular mechanisms underlying the reconsolidationupdating of remote memory are similar to the regulatory mechanisms involved in reopening critical periods of sensory development. If an appropriate visual stimulus is not given at a critical time, even if visual function is restored later, the visual stimulus cannot be seen. However, amblyopia caused by sensory deprivation of one eye is restored through the recovery of visual stimuli along with the reopening of the critical period with drugs (e.g., antidepressants, valproate, ketamine, and psychedelics) (Figure 2) [65,66].
Various psychotropic drugs that affect neuroplasticity have been shown to extend or restore critical periods. Chronic antidepressant treatment and ketamine have also been associated with the reopening of critical periods. Moreover, ketamine can restore amblyopia in adult mice [67]. However, chronic antidepressants can restore amblyopia in younger mice but not in adult mice [68].
The regulatory mechanism of the critical period can be partially explained by the excitatory-inhibitory imbalance hypothesis. The excitatory-inhibitory balance is shifted to a hyperexcitable state through the reopening of the critical period because of decreased GABAergic pathway activity (i.e., through antidepressants and HDAC2 inhibition) and increased excitatory glutamatergic pathway activity (i.e., through ketamine) [65]. The perineural network, which consists of GABA interneurons, is transformed into an immature form with chronic antidepressant use [29] and HDAC2 activity [69] and remains relevant to fear conditioning because this structure also exists in the amygdala, hippocampus and prefrontal cortex [29,66].
If HDAC2 functions as a molecular brake that suppresses the critical period, it would be reasonable to investigate whether HDAC2 inhibition can promote psychotherapeutic effects related to memory reconsolidation (Figure 2). Amblyopia in adult mice can be restored using valproate to reopen the critical period [70]. Valproate was effective in acquiring absolute pitch through training in adults [71]. Valproate combined with growth factors restored hearing loss in a rodent model of sensorineural hearing loss [72]. HDAC2 inhibition is thought to result in “juvenile-like induced plasticity” in the brain, given that the deletion of HDAC2 prolongs the critical period in the visual cortex of mice [69].
Notably, if either of these two factors—critical period reopening and appropriate environmental stimuli—are missing, no restoration of function can be expected. Although several studies have investigated whether valproate has anxiolytic effects [33,37,73,74], whether a combination of CBT and valproate can treat anxiety disorders has not been studied [57].
Off-label use of valproate in panic disorder
In Korea, owing to off-label use regulations in place since 2010, clinical research on the off-label use of psychotropic drugs has decreased significantly [75]. For off-label use research, the Korean Food and Drug Administration (KFDA) requires an investigational new drug (IND) application, which neces sitates proprietary information provided only by the pharmaceutical company. As the patent for valproate has expired, there is little incentive for pharmaceutical companies to participate in research to acquire new indications for their drugs. Many hospital institutional review boards require researchers to obtain IND approvals from the KFDA before submitting a clinical research review. IND approval requires sufficient medical evidence, which includes evidence from two or more placebocontrolled studies, prospective observational studies, or domestic or international medical evidence-based guidelines [76]. Therefore, Korean researchers are faced with a dilemma, as the lack of clinical evidence required research, but clinical evidence was required to obtain approval to perform off-label research.
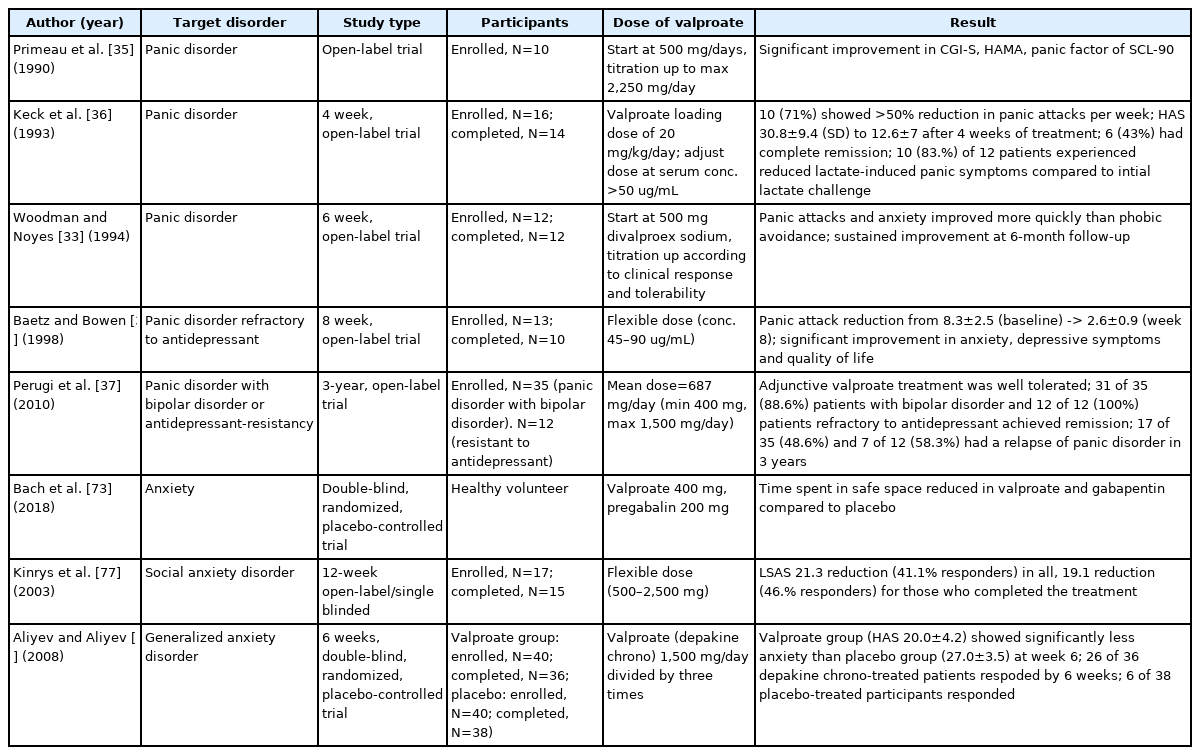
Only five open-label studies have shown promising results in treating patients with panic disorder [33,35,36], panic disorder with comorbid bipolar disorder [37], and antidepressant-refractory panic disorder (Table 2) [37,38]. Even when the search was widened to include anxiety disorder, only one double-blind, placebo-controlled randomized study reported positive results for generalized anxiety disorder [74]. One open-label study showed clinical effects in patients with social anxiety disorder [77]. Furthermore, as there were positive results in only three open-label studies regarding panic disorder, it is difficult to conduct an off-label clinical study with the approval of the KFDA in Korea. To circumvent these obstacles, it is imperative to build evidence to apply valproate adjuvant therapy for CBT for the treatment of panic disorder with comorbid bipolar disorder.
Valproate has been investigated as an alternative to benzodiazepines for anxiety treatment. Valproate 500 mg showed anti-anxiety efficacy in a placebo-controlled randomized study using a computer game designed to test incentive-based risktaking behaviors and risk-avoidance tendencies in healthy volunteers (Table 2) [34]. Unlike benzodiazepines, patients do not develop tolerance to valproate [78]. Furthermore, one study reported a therapeutic effect when a mood stabilizer was administered to patients with anxiety disorders who did not respond to conventional anti-anxiety treatment [34]. Thus, panic disorders that do not respond to antidepressants may be related to a predisposition to bipolar disorder. The effect of valproate facilitated extinction in a preclinical fear conditioning model (Figure 3) [44,45].
Therefore, panic disorder and comorbid bipolar disorder are the first clinical targets to be considered when applying valproate and CBT [34].
Plasticity-augmented psychotherapy
Psychedelic drugs have recently gained increasing attention in psychiatry. Psilocybin-assisted psychotherapy shows promise in the treatment of major depressive disorders [79] and PTSD [80]. Ketamine is attracting attention as a treatment for treatmentresistant depression [81] and suicidal ideation [82]. The mechanisms of these psychedelic and conventional psychoactive drugs converge into enhanced neuroplasticity involving neurotransmitters, neurotrophic factors, and epigenetic modifications (Figure 2) [57,65,83]. These psychedelic drugs affect neuroplasticity with even higher potency than conventional antidepressants [65,84].
Beyond the extensive attention, although efficacy has been demonstrated in well-designed randomized controlled trials for severe refractory major depressive disorder and PTSD [79,80], psychedelic drugs are designed for use only in severely refractory patients during very limited sessions by certified psychotherapists, and there is a risk of substance abuse and uncertainty regarding long-term consequences [85].
From this perspective, the author believes that investigations as to whether valproate, which has been used in clinical practice for a long time and is free from the risk of drug abuse, is effective in enhancing the effects of CBT should be a priority.
How to combine valproate with cognitive behavioral therapy
The optimal dosing strategy for using valproate during CBT remains unknown. The serum concentration of valproate should be at least 36 μg/mL for mild HDAC2 inhibition and 72–120 μg/mL for strong HDAC2 inhibition [86]. The therapeutic range (50–100 μg/mL) for the treatment of bipolar disorder is sufficient for the epigenetic effect to take place. Similar to propranolol [28], intermittent administration of valproate immediately before a psychological intervention is not suitable for clinical application in anxiety disorders. A single intravenous infusion (valproate 30 mg/kg/day) for 60 minutes takes 2–4 hours to reach the peak serum concentration, and a robust HDAC2 inhibition effect (in peripheral lymphocytes) is apparent from 6 to 48 hours after injection according to the results from a phase I clinical trial (valproate adjuvant therapy for chemotherapy for refractory cancer) [87]. At least 2 hours after injection seems to be required for HDAC inhibition to take an effect according to in vitro and in vivo preclinical studies [88,89].
Rapid titration of oral valproate (divalproex sodium) and its maintenance during intensive psychiatric interventions will be adequate for most clinical studies. For the absolute pitch training experiment, valproate ER 500 mg for 3 days and then 1,000 mg for 11 days (total 14 days) during training was used [71]. Valproate 750–1,000 mg was a good starting point. Monitoring the serum valproic acid level could be helpful to adjust the individualized dosage and to check compliance.
The duration of valproate prescription after CBT termination is another question to be investigated. Case 1 received valproate at the start of CBT, and valproate was not discontinued after the termination of CBT for the maintenance treatment of bipolar disorder. Case 2 had already received 300 mg of valproate three years before CBT, and valproate was continued after the termination of CBT. Case 3 was not prescribed valproate before CBT; valproate had been prescribed transiently combined with CBT and was discontinued after the termination of CBT with maintenance treatment of quetiapine and lithium (valproate combined with CBT). If a patient was already on effective maintenance treatment other than valproate, transient use of valproate during CBT could be an option, as in case 3 (Figure 1). If a patient received valproate as a maintenance treatment, a combination of CBT and ongoing valproate after CBT would be rational. Whether transient use of valproate combined with CBT (case 3) would be better than the maintenance of valproate after combined therapy (case 1) or chronic valproate therapy combined with CBT (case 2) remains to be answered.
The same issue has been debated in combined therapy of pharmacotherapy and CBT for anxiety disorders [90-93]. Concurrent anxiolytics such as benzodiazepines have been associated with reduced long-term outcomes of CBT in anxiety disorders [93]. Dose-dependent suppression of cortisol by benzodiazepines [94] is partially responsible for the decreased effect of CBT [91]. Cortisol release following emotional arousal, which occurs during CBT, is known to facilitate extinction learning and exposure-based therapy [91,95]. Antidepressant and CBT combination therapy showed beneficial clinical effects compared to CBT or antidepressants alone in the long-term follow-up of antidepressant treatment [96,97]. However, the beneficial effects of combined therapy appear to disappear after antidepressant discontinuation [97]. According to state-dependent learning theory, taking antidepressants acts as a context, and the behavior learned during antidepressant treatment is not recalled well in an environment where an antidepressant is discontinued [98]. Avoiding safety signals during exposure therapy is in the same vein. Without a safety signal, patients reexperience anxiety because they learn from exposure-based therapy that they are safe while the safety signal is present.
CONCLUSION AND FUTURE DIRECTION
In conclusion, patients with bipolar disorder, comorbid panic disorder, and/or agoraphobia may respond to CBT combined with valproate therapy. CBT should be considered, especially in patients with panic disorder who do not respond to antidepressant treatment. In the future, an open-label or placebocontrolled randomized controlled study is needed to confirm this approach to enhance the efficacy of CBT. If the limited use of valproate during CBT is effective in terms of having lasting effects on anxiety symptoms, the use of valproate in combination with CBT may also be applicable to patients with panic disorder in general.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The author has no potential conflicts of interest to disclose.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2019M3E5D1A02068548).