Differential Gene Expression in the Hippocampi of Nonhuman Primates Chronically Exposed to Methamphetamine, Cocaine, or Heroin
Article information
Abstract
Objective
Methamphetamine (MA), cocaine, and heroin cause severe public health problems as well as impairments in neural plasticity and cognitive function in the hippocampus. This study aimed to identify the genes differentially expressed in the hippocampi of cynomolgus monkeys in response to these drugs.
Methods
After the monkeys were chronically exposed to MA, cocaine, and heroin, we performed large-scale gene expression profiling of the hippocampus using RNA-Seq technology and functional annotation of genes differentially expressed. Some genes selected from RNA-Seq analysis data were validated with reverse transcription-quantitative polymerase chain reaction (RT-qPCR). And the expression changes of ADAM10 protein were assessed using immunohistochemistry.
Results
The changes in genes related to axonal guidance (PTPRP and KAL1), the cell cycle (TLK2), and the regulation of potassium ions (DPP10) in the drug-treated groups compared to the control group were confirmed using RT-qPCR. Comparative analysis of all groups showed that among genes related to synaptic long-term potentiation, CREBBP and GRIN3A were downregulated in both the MA- and heroin-treated groups compared to the control group. In particular, the mRNA and protein expression levels of ADAM10 were decreased in the MA-treated group but increased in the cocaine-treated group compared to the control group.
Conclusion
These results provide insights into the genes that are upregulated and downregulated in the hippocampus by the chronic administration of MA, cocaine, or heroin and basic information for developing novel drugs for the treatment of hippocampal impairments caused by drug abuse.
INTRODUCTION
The use of illicit drugs such as methamphetamine (MA), cocaine, and heroin causes severe public health problems, including anxiety, depression and hallucinations, and has social consequences, such as criminality and mortality [1,2]. Like cocaine, MA is a psychostimulant, but MA is more abused than cocaine because it is cheaper. The repeated use of MA causes neurotoxicity in the hippocampus followed by disruption of the neurotransmitter system and neural plasticity [3]. Some studies have reported that the chronic use of MA causes structural and functional abnormalities in the hippocampus followed by a decrease in neurogenesis [4-6]. In our previous study [7], chronic MA administration in monkeys led to structural atrophy in the hippocampus and changes in various genes, including those related to neurogenesis and synaptic transmission. Furthermore, other research has demonstrated that chronic MA administration causes cognitive impairment as well as neurodegeneration in the hippocampi of rats [8]. Therefore, the chronic use of MA is very harmful to the hippocampus at the structural, cellular, and functional levels.
Cocaine gives rise to the accumulation of monoamine neurotransmitters in the brain by inhibiting their reuptake [9]. The repeated cocaine use causes cocaine-induced neuroadaptations in the brain followed by drug seeking habits and cognitive, motivational, and emotional changes in the brain and ultimately leads to cocaine addiction [10]. Recently, some groups identified changes in genes involved in the inhibition of the mitochondrial inner membrane and GABAergic system in the hippocampi of postmortem cocaine-dependent individuals by performing large-scale transcriptome profiling [11,12]. However, the dosage and duration of use of cocaine and race of the cocaine-dependent individuals used in these studies were not controlled for. Therefore, it is necessary to investigate changes in genes in the hippocampus and the biological functions of these genes upon exposure to cocaine under controlled conditions.
Heroin, an opioid drug, induces euphoric feelings and pain relief. Its repeated use is responsible for depression and negatively affects cognitive function, impulse control, learning, and memory [13,14]. Some studies have reported that the chronic use of heroin induces the elevation of oncoproteins and reduces the level of dopamine D2 receptor in the hippocampi of animal models, suggesting the development of heroin addiction and high motivation for heroin [15,16]. Recently, our team found that even a single injection of heroin induces expression changes in various genes in the hippocampi of long-tailed cynomolgus macaques (also known as Macaca fascicularis) [17]. However, considering that heroin addiction causes serious social and health problems and in particular, brain damage, and because there have only been a few studies related to heroin outside of heroin-related drug seeking studies, the cellular and molecular mechanisms involved in the impairment of brain regions due to heroin addiction should be investigated.
ADAM10, a metalloproteinase, plays various roles in the developing and adult brain. ADAM10 regulates neurite outgrowth during ganglion cell differentiation in the developing brain [18]. In addition, ADAM10 has been reported to be responsible for axon formation, synaptic plasticity, neuronal differentiation, and learning [19]. With regard to its pathophysiological roles, ADAM10 is involved in prion diseases, Huntington’s disease, autism, bipolar disorder, and in particular, Alzheimer’s disease (AD) [19]. Recently, ADAM10 has rapidly emerged as a candidate drug target for AD, a neurodegenerative disease that results from extracellular amyloid-beta deposits. ADAM10, as the major α-secretase, reduces the generation of amyloid-beta peptides by cleaving amyloid precursor protein (APP) [20-22]. Based on previous observations of the pathophysiological roles of ADAM10, studies investigating whether addictive drugs affect diseases caused by changes in the expression of ADAM10 are needed.
As described above, although the psychophysiological effects of MA, cocaine, and heroin in the brain are different, we cannot be sure whether their biomolecular effects on the brain are similar. This study investigated the effect of MA, cocaine, and heroin on gene expression changes in the hippocampi of cynomolgus monkeys and identified the biological functions of genes showing expression changes in the hippocampus and candidate biomarker genes for the diagnosis of neurological diseases caused by drug addiction. The genome sequence of the cynomolgus monkey has 92.8% similarity to that of humans, and the mesocortical dopaminergic system, neuroanatomical structures, and neural circuits of this monkey are similar to those of humans. Therefore, these monkeys have recently been widely used as nonhuman primate animal models to study addiction and neurodegenerative diseases [7,23-25]. We performed large-scale gene expression profiling in the hippocampi of monkeys after chronic administration of MA, cocaine, or heroin and analyzed gene functional annotation and regulatory networks. Then, we identified the expression changes in ADAM10 in the hippocampus at the mRNA and protein levels.
METHODS
Animals
Ten female cynomolgus monkeys (5–7 years of age) with no history of previous participation in drug studies were included in this study. The monkeys originated from Suzhou Xishan Zhongke Laboratory Animal Co. (Suzhou, China) and were housed in individual indoor cages at the National Primate Research Center in Korea Research Institute of Bioscience and Biotechnology (KRIBB) as described previously [26]. All procedures were approved by the KRIBB Institutional Animal Care and Use Committee (Approval No. KRIBB-AEC-15046).
Drug treatment
To perform the experiments, the 10 monkeys were randomly divided into 3 groups: the control group (n=3), cocaine-treated group (n=4), and heroin-treated group (n=3). The control group was intramuscularly injected with 0.1 mL of 0.9% saline for 10 weeks. Cocaine hydrochloride (Johnson Matthey Macfarlan Smith, Edinburgh, Scotland) was freshly dissolved in 0.9% saline immediately before administration. Heroin, also known as diamorphine (Johnson Matthey Macfarlan Smith), was freshly dissolved in 0.9% saline immediately before administration. The cocaine- and heroin-treated groups were injected with 0.9% saline for the first 2 weeks and then received drug injections for 8 weeks. The drugs were administered as done in our previous study [27]. MA treatment has been performed in our previous study [7]. To briefly explain MA administration procedure, 4 female monkeys were intramuscularly injected with 0.9% phospate-buffered saline for the first 2 weeks followed by MA for 8 weeks. MA dosage was gradually increased from 0.1 to 0.75 mg/kg for the first 4 weeks and was maintained at 0.75 mg/kg for the last 4 weeks. The MA, cocaine, and heroin administration schedules are presented in Supplementary Figure 1 (in the online-only Data Supplement).
RNA-Seq library preparation and sequencing
Monkeys were sacrificed ten weeks after they were treated with drugs (cocaine [n=4] or heroin [n=3]) or the control (n=3). Total RNA was isolated from the hippocampi of the control and drug-treated animals using TRIzol (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. The integrity of the total RNA was checked using a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), and the RNA integrity number (RIN) of the RNA was greater than 8. For RNA-Seq, RNA libraries were prepared using the TruSeq RNA library preparation kit (Illumina, San Diego, CA, USA) as done in our previous study [7]. The constructed libraries were 101-bp paired-end sequenced using an Illumina HiSeq 2500 sequencer.
Differential gene expression analysis
In this study, we combined RNA-Seq raw data from this study with RNA-Seq raw data obtained after chronic administration of MA in our previous study [7]. The raw reads obtained from RNA-Seq underwent quality control analysis using FastQC (version 0.10.1; FastQC: A Quality Control Tool for High Throughput Sequence Data, 2010; http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). To remove lowquality data and artifacts, including adaptor sequences, contaminant DNA and PCR duplicates, preprocessing of reads was performed using Trimmomatic version 0.32 [28]. The preprocessed reads were mapped into a reference genome (Macaca fascicularis_5.0 in NCBI) using TopHat (version 2.1.0; Center for Computational Biology at Johns Hopkins University; http://ccb.jhu.edu/software/tophat/index.shtml) software, and aligned reads were produced. The transcripts of each sample were assembled by Cufflinks [29] based on the fragments per kilobase of the transcript per million mapped reads (FPKM) method. Transcripts with FPKM values of zero in more than one sample were excluded. To facilitate log2 transformation, 1 was added to each FPKM value of the filtered genes. The filtered data were log2-transformed and subjected to quantile normalization. The statistical significance of the differential expression data was determined using independent t-test and fold changes based on the null hypothesis that there are no differences in the expression levels of genes among groups. The false discovery rate was controlled by adjusting the p-value (p<0.05) using the Benjamini–Hochberg algorithm. For the differentially expressed gene (DEG) set among control and drug (MA, cocaine, and heroin)-treated groups, hierarchical clustering analysis was performed using complete linkage and Euclidean distance as a measure of similarity of the expression patterns of differentially expressed transcripts while satisfying the conditions fold change ≥1.5 and independent t-test raw p<0.05. All data and visualization of DEGs were conducted using R 3.1.2 (R Development Core Team, 2013; http://www.r-project.org).
Functional annotation and pathway analysis
Gene ontology (GO) and network pathway analyses were performed using the UniProt database (M. fascicularis shares more than 92% sequence homology with humans). The DAVID 6.8 tool (DAVID Bioinformatics Resources; https://david.ncifcrf.gov) was used for functional annotation and enrichment analysis of genes that were differentially expressed in response to MA, cocaine, and heroin. Statistically overrepresented GO categories at p<0.05 were considered significant. To further analyze biological responses and various canonical pathways associated with DEGs, ingenuity pathway analysis (IPA) software (Ingenuity System, Redwood City, CA, USA) was employed. IPA identified cellular networks in which DEGs were related based on previously known associations between genes or proteins but independent of established canonical pathways.
Reverse transcription-quantitative polymerase chain reaction
To validate the differential expression of some of the genes identified by RNA-Seq, we performed reverse transcriptionquantitative polymerase chain reaction (RT-qPCR). To validate genes differentially expressed in the MA-treated group using RT-qPCR, we used hippocampal tissues from MA-treated animals (n=4) that had been stored at -80°C after being used in our previous study [7]. The tissues had been immersed in Trizol reagent (Thermo Fisher Scientific, San Jose, CA, USA) to prevent RNA degradation. Total RNA extracted from the hippocampi of control, MA-, cocaine-, and heroin-treated animals was reverse transcribed into cDNA using the Superscript RT III system (Thermo Fisher Scientific). Details of the qPCR method have been described previously [30]. Three independent qPCR experiments were performed to guarantee reliable results for all samples from each group (the control, MA-, cocaine- and heroin-treated groups). The expression of the DEGs in each sample was normalized to GAPDH expression. The relative expression differences among the control (n=3), MA-treated (n=4), cocaine-treated (n=4), and heroin-treated (n=3) groups were calculated using the 2-ΔΔCT method. The primers used for the amplification of the candidate genes are presented in Supplementary Table 1 (in the online-only Data Supplement).
Immunohistochemistry
Hippocampal tissues collected from control, cocaine-treated, and heroin-treated monkeys were fixed in 4% formaldehyde for 24 h, washed with distilled water, and dehydrated gradually with a series of 70%–100% ethanol solutions. The tissues were immersed in xylene, embedded in paraffin, and sliced into 3-μm sections. The obtained sections, including sections obtained from paraffin blocks of MA-treated monkeys from our previous study [7], were transferred onto slides. Detailed methods of immunohistochemistry (IHC) and ADAM10 intensity measurement are described in the Supplementary Material (in the online-only Data Supplement).
Statistical analysis
Statistical analyses were conducted with SPSS 18.0 software (IBM Co., Armonk, NY, USA) and GraphPad Prism 8 software (San Diego, CA, USA). All data obtained from RT-qPCR and IHC are expressed as the mean±standard errors of the mean. Differences between the control, MA-, cocaine-, and heroin-treated groups were analyzed by a one-way analysis of variance and Tukey’s honestly significant difference test was performed to determine a statistically significant difference between specific groups. p<0.05 was considered statistically significant.
RESULTS
Identification and functional annotation of differentially expressed genes
To investigate the effects of MA, cocaine, and heroin on gene expression patterns in the hippocampus, we performed large-scale transcriptome profiling by combining RNA-Seq data from chronic MA-treated monkeys produced in our previous study [7] and RNA-Seq data from animals chronically exposed to cocaine and heroin obtained in this study. After mining the data from the control, MA-, cocaine-, and heroin-treated groups using log2 transformation (fold change cutoff of 1.5), a heat map of 10,752 transcripts was constructed using hierarchical clustering analysis (Figure 1A). The strongest correlation of gene expression among all analyzed groups was found between the control and heroin-treated groups. When comparing the drug-treated groups, the gene expression pattern in the cocaine-treated group was opposite that in the MA-treated group, even though both drugs are psychostimulants. In order to show overlapped or specific transcripts among comparison pairs (MA vs. control, cocaine vs. control, heroin vs. control, MA vs. cocaine, cocaine vs. heroin, and MA vs. heroin), we created Venn diagrams. Of the 10,752 transcripts, 1,846, 1,608, and 772 transcripts were upregulated or downregulated more than 1.5-fold in the MA-, cocaine-, and heroin-treated groups, respectively, compared to the control group (Figure 1B). One hundred three transcripts were upregulated or downregulated in the MA-, cocaine-, and heroin-treated groups compared to the control group. On the other hand, when comparing the genes expressed differentially among the MA-, cocaine-, and heroin-treated groups, 3,088 and 481 transcripts were upregulated or downregulated more than 1.5-fold in the MA- and cocaine-treated groups, respectively, compared to the heroin-treated group (Supplementary Figure 2A in the online-only Data Supplement).
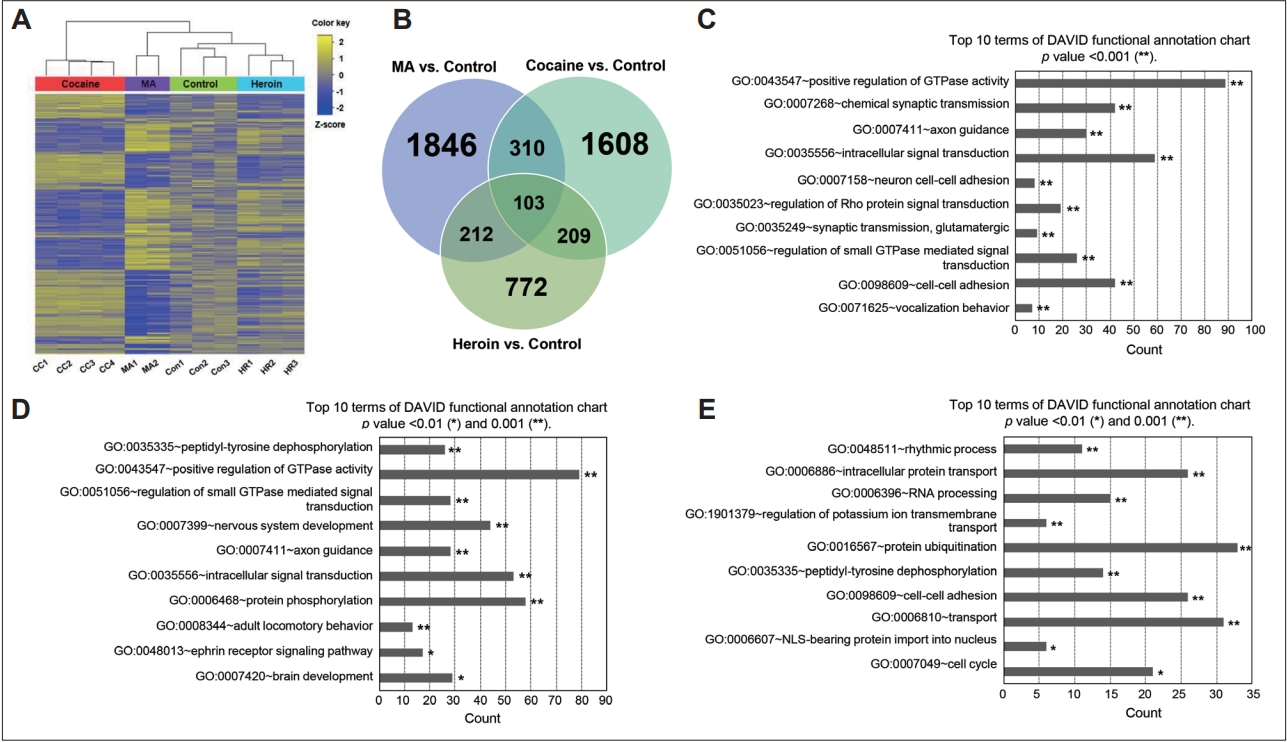
Gene expression changes in response to MA, cocaine, or heroin and GO annotation. A: Heatmap of two-way hierarchical clustering analysis of DEGs among the control and MA-, cocaine-, and heroin-treated groups. B: Venn diagram showing the overlap of DEGs among the control and MA-, cocaine-, and heroin-treated groups. C: Top 10 enriched terms in the BP category for genes expressed differentially in the MA-treated group compared to the control group. D: Top 10 enriched terms in the BP category for genes expressed differentially in the cocaine-treated group compared to the control group. E: Top 10 enriched terms in the BP category for genes expressed differentially in the heroin-treated group compared to the control group. MA, methamphetamine; GO, gene ontology; DEG, differentially expressed gene; BP, biological process.
We performed GO-based functional annotation of DEGs and classified the GO categories into 3 types (biological process [BP], cellular component, and molecular function). The categories were subsequently subdivided into hyperlinked GO categories using GO terms. Based on the BP category, genes associated with the positive regulation of GTPase activity, chemical synaptic transmission, and axon guidance were differentially expressed in the MA-treated group compared to the control group (Figure 1C). Genes involved in peptidyl-tyrosine dephosphorylation, the positive regulation of GTPase activity, and nervous system development were differentially expressed in the cocaine-treated group compared to the control group (Figure 1D). On the other hand, genes involved in rhythmic process and intracellular protein transport were differentially expressed in the heroin-treated group compared to the control group (Figure 1E). When comparing the MA- and cocaine-treated groups, genes related to the positive regulation of GTPase activity and protein phosphorylation were differentially expressed (Supplementary Figure 2B in the online-only Data Supplement). When comparing the MA- and heroin-treated groups, genes related to chemical synaptic transmission and the positive regulation of GTPase activity were differentially expressed (Supplementary Figure 2C in the online-only Data Supplement). Genes involved in peptidyl-tyrosine dephosphorylation and the positive regulation of GTPase activity were differentially expressed between the cocaine- and heroin-treated groups (Supplementary Figure 2D in the online-only Data Supplement).
Pathway network identification
To better understand the biological and cellular mechanisms and pathways related to the DEGs among all tested groups, we further analyzed the DEGs using IPA software. When comparing DEGs between the MA-treated and control groups, 25 networks were identified (Supplementary Table 2 in the online-only Data Supplement), and the top-ranked network included 33 focus genes related to neurological disease, organismal injury and abnormalities, and psychological disorders (IPA score, 31; Figure 2A). Twenty-five networks were also identified between the cocaine-treated and control groups (Supplementary Table 2 in the online-only Data Supplement), and the top-ranked network included 32 focus genes related to gastrointestinal disease, hepatic system disease, and organismal injury and abnormalities (IPA score, 30; Figure 2B). On the other hand, of the 25 networks identified between the heroin-treated and control groups (Supplementary Table 2 in the online-only Data Supplement), the top network included 33 focus genes related to embryonic development, organismal development, and nervous system development and function (IPA score, 39; Figure 2C). In addition, when comparing the MA-treated and cocaine-treated groups, the top-ranked network (IPA score, 23) included 35 focus genes involved in cell-to-cell signaling and interaction, cellular function and maintenance, and inflammatory response (Supplementary Figure 3A and Supplementary Table 2 in the onlineonly Data Supplement). The top-ranked network (IPA score, 31) identified by comparing the MA-treated and heroin-treated groups included 33 focus genes related to drug metabolism, molecular transport, and small molecule biochemistry (Supplementary Figure 3B and Supplementary Table 2 in the online-only Data Supplement). On the other hand, the top-ranked network (IPA score, 25) identified by comparing the cocaine- and heroin-treated groups involved 35 focus genes related to cellular movement, cellular development, and lipid metabolism (Supplementary Figure 3C and Supplementary Table 2 in the online-only Data Supplement).
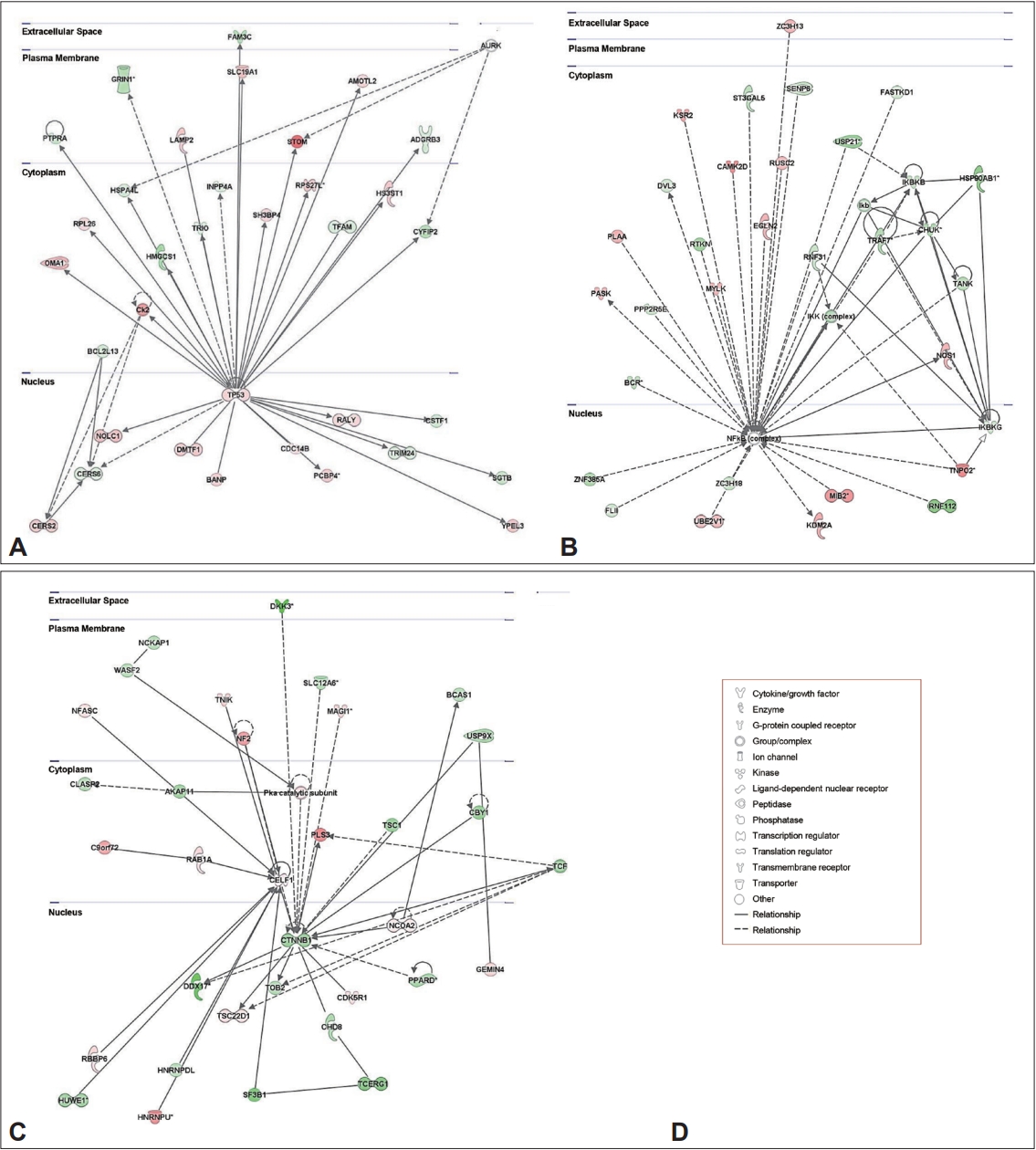
Top networks identified using ingenuity pathway analysis of genes regulated by MA, cocaine, or heroin. A: The top network of genes expressed differentially in the MA-treated group compared to the control group. B: The top network of genes expressed differentially in the cocaine-treated group compared to the control group. C: The top network of genes expressed differentially in the heroin-treated group compared to the control group. The intensity of the node (gene or gene products) color indicates the degree of upregulation (red) or downregulation (green). D: Node legend. MA, methamphetamine.
When we investigated canonical pathways involved in the control and drug-treated groups by comparative analysis, more than 130 canonical pathways were identified. Of 130 canonical pathways, 30 pathways were selected by sorting the pathways based on z-score (Figure 3A). When comparing the pathways involving genes differentially expressed among the MA-, cocaine-, and heroin-treated groups, we found that FLT3 signaling in hematopoietic progenitor cells was downregulated in both the MA- and heroin-treated groups but upregulated in the cocaine-treated group. In addition, most pathways were downregulated in both the MA- and heroin-treated groups compared to the control group, while other pathways, except PI3K/AKT signaling, were upregulated in the cocaine-treated group compared to the control group. When we evaluated synaptic long-term potentiation (LTP) in detail, glutamate receptors, some of the downstream factors of these receptors, and CREB were downregulated in postsynaptic neurons in the MA- and heroin-treated groups but were upregulated in postsynaptic neurons in the cocaine-treated group compared to the control group (Figure 3B-D). Based on these results, it can be speculated that among the three drugs, MA and heroin affect canonical pathways in the hippocampus in a similar manner relative to cocaine.
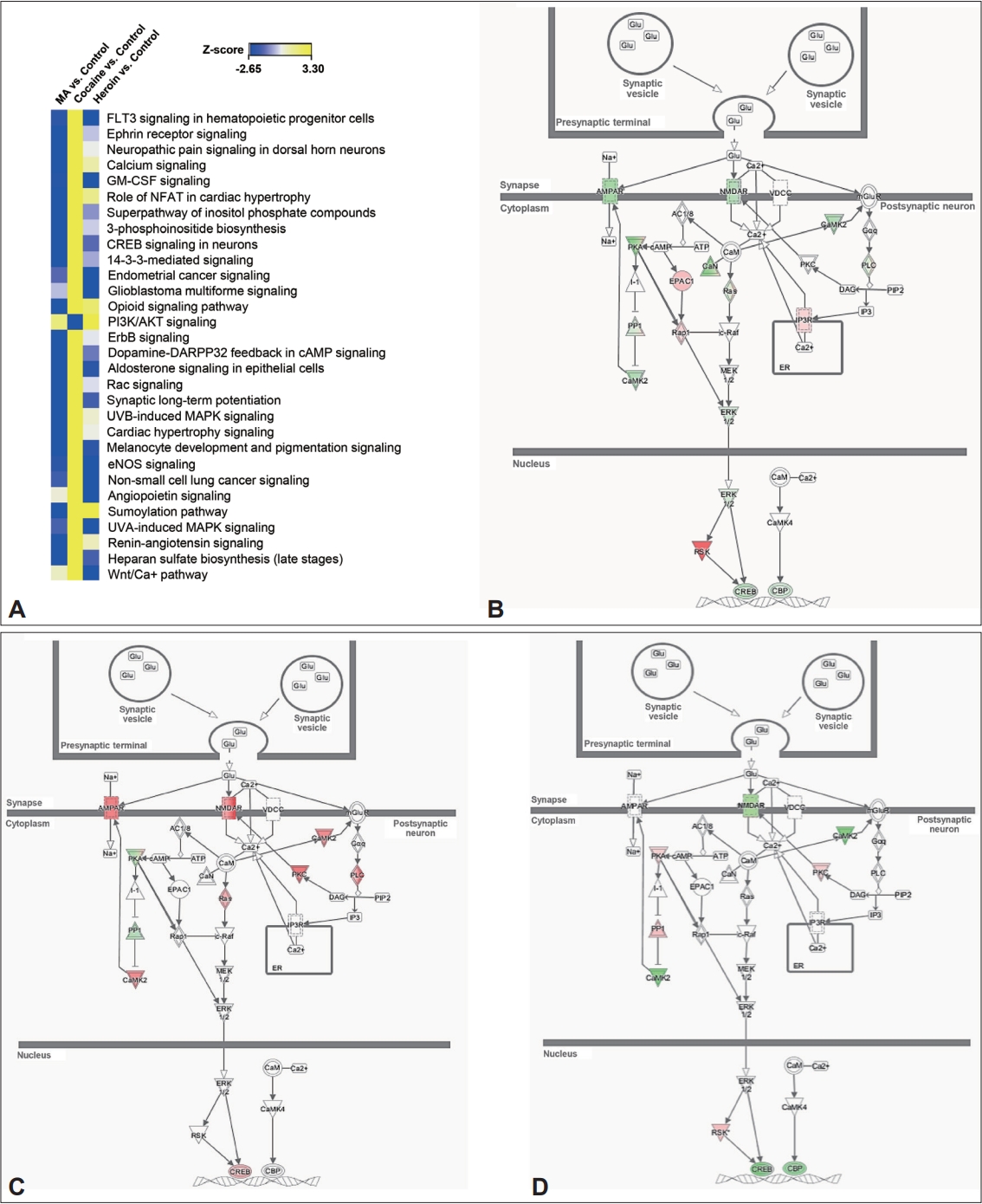
Identification of canonical pathways regulated by chronic treatment with MA, cocaine, or heroin. A: The top 30 canonical pathways identified by comparative analysis between the control and drug-treated groups. B: Synaptic long-term potentiation in the control and MA-treated groups. C: Synaptic long-term potentiation in the control and cocaine-treated groups. D: Synaptic long-term potentiation in the control and heroin-treated groups. The intensity of the node (gene or gene products) color indicates the degree of up- (red) or down- (green) regulation. MA, methamphetamine.
Differentially expressed gene validation based on reverse transcription-quantitative polymerase chain reaction
To confirm the expression changes of genes identified to be differentially regulated by RNA-Seq analysis, we selected 15 genes based on gene function annotation and IPA network analysis (Figure 4A) and validated them using RT-qPCR. Among them, PTPRO and KAL1, which are related to axon guidance, were significantly downregulated in the MA-treated group but significantly upregulated in the cocaine-treated group compared to the control group (Figure 4B). ADAM10, which is related to the ephrin receptor signaling pathway, was significantly decreased in both the MA- and heroin-treated groups but significantly increased in the cocaine-treated group compared to the control group. Among cell cycle-related genes, CCDC124 was significantly upregulated in the MA- and heroin-treated groups compared to the control group, while TLK2 was significantly downregulated in the MA- and heroin-treated groups, showing an expression pattern consistent with that shown by the RNA-Seq results. In addition, DPP10 (related to the regulation of potassium ions) and ATP6V1A (related to transport) were significantly downregulated in the MA- and heroin-treated groups but significantly upregulated in the cocaine-treated group compared to the control group. CTNNB1 and USP9X were significantly downregulated in both the MA-and heroin-treated groups compared to the control group (Figure 4C). DVL3 and RBM3 were significantly downregulated in all drug-treated groups compared to the control group. On the other hand, DDX3X was significantly reduced in the MA-and heroin-treated groups compared to the cocaine-treated and control groups.
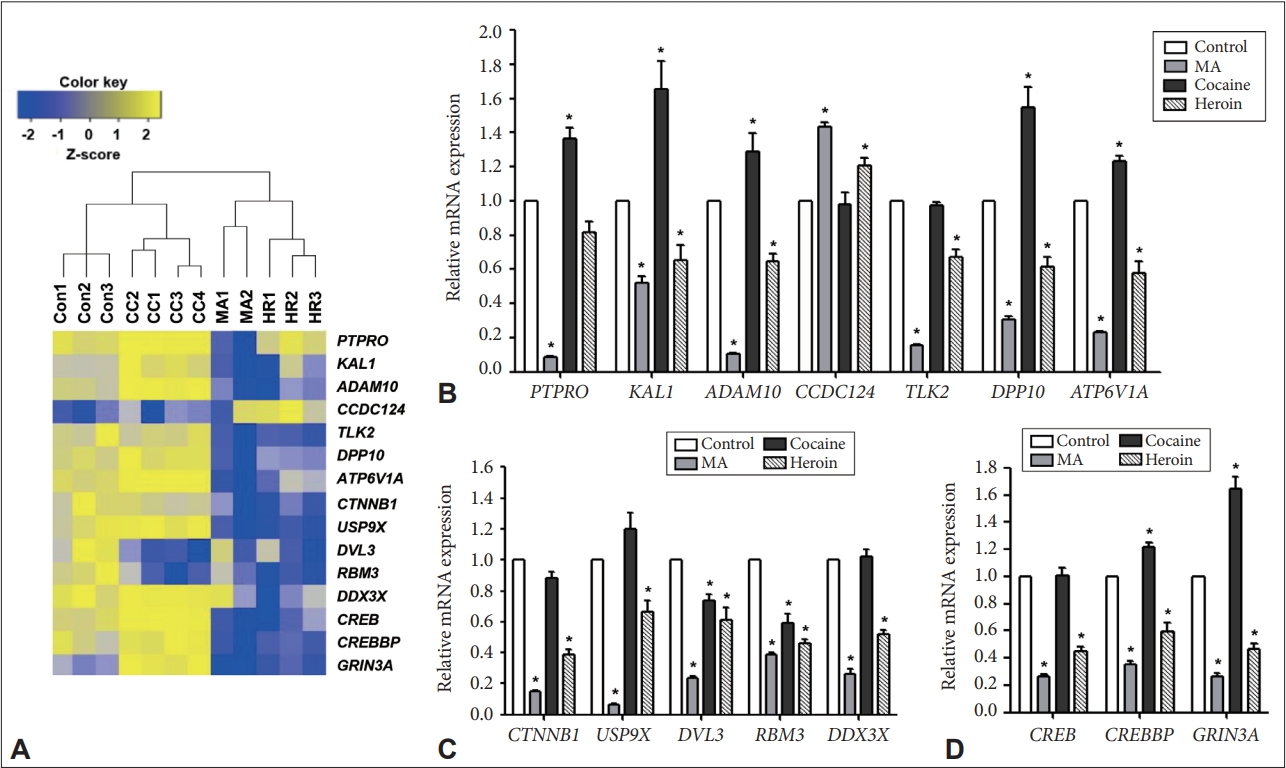
Validation of some of the genes identified using RNA-Seq. After monkeys were chronically exposed to MA, cocaine, or heroin, total RNA was extracted from the hippocampus and analyzed by reverse transcription-quantitative polymerase chain reaction. A: Heatmap of the genes identified after normalizing the expression values of the genes selected from RNA-Seq data and converting the values to z-scores. B: The expression levels of genes involved in axon guidance (PTPRO and KAL1), the ephrin receptor signaling pathway (ADAM10), the cell cycle (TLK2), the regulation of potassium ions (DPP10), and transport (ATP6V1A). C: The expression levels of genes selected from the top networks involved in the MA-treated (n=4), cocaine-treated (n=4), and heroin-treated (n=3) groups compared to the control group (n=3). D: The expression levels of genes involved in synaptic long-term potentiation. *Significantly different from the control (p<0.05). MA, methamphetamine.
The differential expression of 3 genes involved in synaptic LTP among the control and MA-, cocaine-, and heroin-treated groups identified by comparative analysis, was validated using RT-qPCR. The CREB, CREBBP, and GRIN3A genes were significantly downregulated in both the MA- and heroin-treated groups compared to the control group, while CREBBP and GRIN3A were significantly upregulated in the cocaine-treated group compared to the control group (Figure 4D). The expression patterns of all the validated genes were similar to those observed in RNA-Seq.
Change in ADAM10 protein in the hippocampus
Based on the decrease in the ADAM10 gene in the MAand heroin-treated groups and the increase in the ADAM10 gene in the cocaine-treated group observed in this study, we further investigated the expression changes in the ADAM10 protein in the hippocampus using IHC. Among all observed groups, the cocaine-treated group exhibited the highest expression of ADAM10 in all areas of the hippocampus (Figure 5A-D). The expression density of ADAM10 was significantly (p=0.008) reduced in the MA-treated group but significantly (p=0.026) elevated in the cocaine-treated group compared to the control group (Figure 5E). On the other hand, the expression of ADAM10 tended to be lower (p=0.095) in the heroin-treated group than in the control group. Therefore, chronic exposure to MA or cocaine induces changes in the expression of ADAM10 in the hippocampus at both the mRNA and protein levels.

Changes in the expression of ADAM10 in the hippocampus. Hippocampi were obtained after the chronic administration of MA, cocaine, or heroin, and the expression levels of ADAM10 in the hippocampus were analyzed using immunohistochemistry. A-D: ADAM10 expression in the control, MA-, cocaine-, and heroin-treated groups, respectively. Scale bar=500 µm (insert: 50 µm). E: Densitometric analysis of the relative intensity of ADAM10 expression in the hippocampus. The data are represented as the mean±standard error of the mean of 3 monkeys per group. *Significantly different from the control group (p<0.05). MA, methamphetamine.
DISCUSSION
Repeated exposure to addictive drugs such as MA, cocaine, and heroin causes impairments in the brain, particularly the hippocampus, as well as the disruption of neurotransmitter systems [3,9,14]. In addition, it is necessary to investigate changes in processes caused by addictive drugs to understand their psychophysiological effects. Therefore, we established animal models of chronic MA, cocaine, and heroin administration in cynomolgus monkeys and performed profiling of genes expressed differentially in the hippocampus among the MA-, cocaine-, and heroin-treated groups.
In this study, GO annotation enrichment identified changes in genes related to axon guidance in the drug-treated groups; among these genes, KAL1 gene was downregulated in the MAand heroin-treated groups but upregulated in the cocainetreated group. Genes related to axon guidance can be ligands or receptors that guide axons and initiate signaling pathways related to proper neural circuit formation. Axon guidance ligands mainly interact with receptors that are expressed on growth cones, and ligand/receptor complexes promote intracellular signaling cascades for axon guidance. Defects in proteins involved in axon guidance may cause pathological changes in neural circuits. KAL1 decreased upon treatment with MA or heroin in our study encodes the extracellular glycoprotein anosmin-1 and is mainly expressed in embryonic tissues, including the cerebellum and olfactory bulbs [31]. A previous study demonstrated that anosmin-1 enhances axonal branch formation in olfactory bulb output neurons [32]. Recently, studies have shown that a mutation in KAL1 is involved in Kallmann syndrome, of which congenital anosmia is a symptom [33]. Loss-of-function mutations in KAL1 cause defective olfactory axon guidance, resulting in X-linked Kallmann syndrome [34]. Based on previous studies and our results, a decrease in KAL1 caused by chronic treatment with MA or heroin negatively affects axon guidance. However, considering that chronic cocaine treatment induces an increase in KAL1, cocaine may differentially regulate axon guidance compared to MA and heroin.
Among the cell cycle-related genes identified in the present study, TLK2 was downregulated in both the MA- and heroin-treated groups compared to the control and cocaine-treated groups. The Tousled-like kinase family is composed of TLK1 and TLK2. TLK2 is maximally activated during the Sphase of the cell cycle and cooperates with TLK1 to sustain chromosomal stability and cell viability [35]. A previous study indicated that virally encoded Abeta42, which is the main component of amyloid plaques in AD, represses the phosphorylation of TLK2 in humans, suggesting the possibility that a decrease in phosphorylated TLK2 is correlated with AD [36]. Taken together, it is supposed that the chronic use of MA or heroin leads to a reduction in TLK2 and may further negatively affect cell viability in the hippocampus.
In this study, DPP10 was downregulated in the MA- and heroin-treated groups but upregulated in the cocaine-treated group compared to the control group. DPP10, as a single-pass type II membrane protein that binds to specific voltage-gated potassium channels, regulates various cellular processes, such as neuronal excitability and neurotransmitter release [37]. In the hippocampus, DPP10 is expressed in approximately 6.4% of inhibitory neurons but not in glia [38]. Copy number variation of the DPP10 gene has been reported to be related to autism, which is a neurodevelopmental disease [39]. It has been reported that the short form of DPP10, DPP10789, is abnormally expressed in neurodegenerative diseases, including AD [40]. Based on previous studies showing that DPP10 is associated with neurological diseases and our results, the chronic use of MA, cocaine, or heroin may impair the hippocampus by inducing alterations in DPP10 expression. On the other hand, the protein encoded by ATP6V1A, which showed a similar expression pattern as DPP10 upon chronic exposure to drugs in the present study, is responsible for neurotransmitter release and the acidification of synaptic vesicles after exocytosis [41]. ATP6V1A knockdown in zebrafish induces impairments in acid secretion and ion balance, causing abnormalities such as the loss of internal Ca2+ and Na+, trunk deformation, and growth retardation [42]. In addition, Fassio et al. [43] identified that ATP6V1A expression is increased during the in vitro maturation of neurons derived from the rat hippocampus and synaptogenesis. According to previous studies [42,43] and our results, ATP6V1A plays an important role in cell growth in the hippocampus, and expression changes in ATP6V1A due to chronic exposure to MA, cocaine, or heroin may cause abnormal maturation of neurons in the hippocampus.
In the present study, CTNNB1, which is related to nervous system development and function, was decreased in the hippocampus by the chronic administration of MA or heroin. CTNNB1 encodes β-catenin, which mediates neural circuit formation and synaptic plasticity [44,45]. Several studies have demonstrated that MA inhibits β-catenin signaling in astrocytes, resulting in cellular senescence and neuronal toxicity [46-48]. On the other hand, the mRNA expression of DVL3 was reduced in all drug-treated groups in the present study. DLV3, as a scaffold protein that links the receptor and downstream signaling molecules, is also associated with β-catenin signaling [49]. Previous studies have reported that DVL3 mRNA is reduced in the nucleus accumbens and frontal lobes of individuals with major depressive disorder (MDD) [50,51]. Additionally, it was demonstrated that DVL3 as well as β-catenin are increased in the hippocampi and ventral midbrains of female rats after the chronic administration of antipsychotic drugs, including haloperidol and risperidone [52]. These studies imply that expression change in DVL3 may be associated with neuropsychiatric disorders. Based on previous studies and our results, chronic exposure to MA, cocaine, or heroin induces decreased DVL3, which has negative effects on β-catenin signalingrelated function in the hippocampus.
In the present study, the expression level of DDX3X, which encodes DDX3X, a multifunctional RNA helicase, was decreased in the MA- and heroin-treated groups but was not changed in the cocaine-treated group. DDX3X is responsible for transcription [53], pre-mRNA splicing [54], RNA export [55], and translation [56]. A previous study reported that the inhibition of DDX3X causes impairments in spine formation and neurite outgrowth in hippocampal neurons originating from normal rat brains, showing that DDX3X is essential for spine formation and neurite outgrowth in the brain [57]. In addition, the brains of patients with gliomas exhibit increased DDX3X expression compared to that in normal brains, suggesting that the upregulation of DDX3X may positively affect human glioma progression [58]. Considering that DDX3X enhances the transcription and translation of certain genes regardless of the cell state, as mentioned above, cancer cells can also cause increased expression of DDX3X. However, it has been demonstrated that DDX3X is associated with spine formation and neurite outgrowth in the normal brain, and chronic exposure to MA or heroin causes a reduction in DDX3X in the normal brain, implying that chronic exposure to these drugs may impair neurite outgrowth and spine formation.
In the present study, gene expression patterns in the hippocampus showed the highest correlation between MA- and heroin-treated groups but gene expression patterns of the two groups were opposite to that of cocaine-treated group. This result suggests that MA and heroin have similar effects on the expression change of one gene in the hippocampus, while cocaine has the opposite effect on the expression change of the gene. When exploring canonical pathways involved in the altered genes based on this finding, change patterns of most pathways were also similar to gene expression patterns among groups. In particular, top thirty pathways showed decrease in both MA- and heroin-treated groups but increase in cocaine-treated groups. Therefore, these results imply that in the hippocampus gene expression changes by MA, cocaine, and heroin may affect changes in protein expression and function.
Repeated stimulation that lasts for hours or longer enhances the efficiency of synaptic transmission [59]. The phenomenon that underlies this enhancement is LTP, which is known as memory at the cellular level. Therefore, if synaptic LTP in the CA1 area is impaired, memory function is decreased [60]. Addictive drugs induce changes in LTP in the hippocampus, resulting in impaired synaptic plasticity. Our comparative analysis of DEGs among the MA-, cocaine-, and heroin-treated groups and the control group showed that synaptic LTP-related genes were altered by MA, cocaine, and heroin. Interestingly, compared to the control, MA and heroin mainly caused the downregulation of LTP-related genes, while cocaine mainly induced the upregulation of LTP-related genes. Cocaine enhances LTP in the CA1 of the hippocampus [61,62], while MA, a psychostimulant like cocaine, induces the impairment of LTP [63]. On the other hand, heroin induces reduced LTP in the CA1 region of the hippocampus [64,65]. Based on previous findings and our results showing alterations in or the impairment of LTP by these drugs, we validated the alterations in the expression levels of LTP-related genes (CREB, CREBBP, and GRIN3A) in the drug-treated groups compared to the control group. The expression patterns of these genes in the MA-treated group were the same as those in the heroin-treated group but were the opposite of those in the cocaine-treated group. Therefore, in agreement with previous reports, this study not only confirmed that chronic exposure to MA, cocaine, or heroin induces alterations in synaptic LTP in the hippocampus but also identified expression changes in LTP-related genes induced by MA, cocaine, or heroin.
ADAM10 is associated with the shedding of cell surface proteins required for brain development, such as ephrins [20]. Considering that Adam10 knockout in mice induces prenatal lethality at embryonic day 9.5 as well as defects in the cardiovascular system and developing central nervous system [66], ADAM10 is an essential factor for survival as well as brain development. In the present study, ADAM10 was decreased in the heroin-treated group and showed a particularly drastic reduction in the MA-treated group, but it was significantly increased in the cocaine-treated group. Contrary to our results, an in vitro study reported that the exposure of human neuroblastoma SH-SY5Y cells to 1 μM or 10 μM MA for 16 h induces an increase in the mRNA expression of ADAM10 but that 100 and 1,000 μM MA do not induce any changes [67]. Taken together, these results suggest that ADAM10 is differentially expressed based on the time and condition of MA treatment. As mentioned earlier, ADAM10 not only participates in the cleavage of APP, leading to reduced production of amyloid-β peptides [20-22] but is also involved in neuronal differentiation, axon formation, and synaptic plasticity [19]. Considering that the accumulation of amyloid-β in the brain is a signal of the fundamental neuropathological changes in AD and that the FDA-approved drug library for AD therapy targets increased ADAM10 gene expression [68], expression changes in ADAM10 caused by addictive drugs may affect the development of AD. Based on our results showing that ADAM10 was reduced by heroin and, in particular, the MA, the chronic administration of these two drugs may lead to the development of early-onset AD. However, as cocaine, unlike MA, induced an increase in ADAM10, how the increase in ADAM10 induced by chronic cocaine treatment affects the hippocampus and the development of AD requires further study.
In summary, we profiled the expression of genes upregulated and downregulated by the chronic administration of MA, cocaine, and heroin. We demonstrated changes in genes involved in axon guidance (PTPRO and KAL1), the ephrin receptor signaling pathway (ADAM10), the cell cycle (TLK2), the regulation of potassium ions (DPP10), transport (ATP6V1A), and synaptic LTP (CREB, CREBBP, and GRIN3A). In particular, we determined that chronic MA administration caused a decrease in ADAM10 expression at both the mRNA and protein levels but that chronic cocaine administration caused an increase in ADAM10 expression at both the mRNA and protein levels, showing that ADAM10 is differentially regulated by the administration of MA and cocaine. To the best of our knowledge, this is the first study using large-scale transcriptome profiling of the hippocampi of monkeys exposed to MA, cocaine, or heroin by RNA-Seq. Our findings show both the genes affected by MA, cocaine, and heroin as well as their biological functions. Therefore, these results not only aid in understanding the biomolecular processes in the hippocampus regulated by MA, cocaine, and heroin but also provide novel insight into the etiology of drug addiction and potential targets for developing novel biomarkers for diagnosing or treating hippocampal impairments caused by drug abuse.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2022.0004.
Primers used in reverse transcription-quantitative polymerase chain reaction
Top twenty-five networks obtained from IPA analysis of differentially expressed genes among control, MA-, cocaine-, and heroin-treated groups.
Schematic representation of the experimental procedure for MA, cocaine, and heroin administration to monkeys. MA, methamphetamine.
Genes differentially expressed among the MA-, cocaine-, and heroin-treated groups and GO annotation. A: Venn diagram showing the overlap of DEGs among the MA-, cocaine-, and heroin-treated groups. B: Top 10 enriched terms in the BP category for DEGs in the cocaine-treated group compared to the MA-treated group. C: Top 10 enriched terms in the BP category for DEGs in the herointreated group compared to the MA-treated group. D: Top 10 enriched terms in the BP category for DEGs in the heroin-treated group compared to the cocaine-treated group. MA, methamphetamine; GO, gene ontology; DEG, differentially expressed gene; BP, biological process.
Top networks identified using ingenuity pathway analysis of genes differentially expressed among the MA-, cocaine-, and heroin-treated. A: The top network of genes differentially expressed between the MA- and cocaine-treated groups. B: The top network of genes differentially expressed between the MA- and heroin-treated groups. C: The top network of genes differentially expressed between the cocaine- and heroin-treated groups. The intensity of the node (gene or gene products) color indicates the degree of upregulation (red) or downregulation (green). D: Node legend. MA, methamphetamine.
Notes
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Mi Ran Choi, Yeung-Bae Jin, Sang-Rae Lee, Dai-Jin Kim. Data curation: Mi Ran Choi, Yeung-Bae Jin. Formal analysis: Mi Ran Choi, Young Gyu Chai. Funding acquisition: Sang-Rae Lee, Dai-Jin Kim. Methodology: Yeung-Bae Jin, Han-Na Kim. Project administration: SangRae Lee, Dai-Jin Kim. Supervision: Sang-Rae Lee, Dai-Jin Kim. Validation: Heejin Lee. Writing—original draft: Mi Ran Choi, Yeung-Bae Jin. Writing—review & editing: Sang-Rae Lee, Dai-Jin Kim.
Funding Statement
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation funded by the Korean government, MSIP (NRF-2014M3A9B6070246), the intramural research fund of Ajou University medical center (M2021C046000054), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR21C1003).
