Abelson Helper Integration Site-1 Gene Variants on Major Depressive Disorder and Bipolar Disorder
Article information
Abstract
Objective
The present study aimed to explore whether 4 single nucleotide polymorphisms (SNPs) within the AHI1 gene could be associated with major depressive disorder (MD) and bipolar disorder (BD), and whether they could predict clinical outcomes in mood disorders.
Methods
One hundred and eighty-four (184) patients with MD, 170 patients with BD and 170 healthy controls were genotyped for 4 AHI1 SNPs (rs11154801, rs7750586, rs9647635 and rs9321501). Baseline and final clinical measures for MD patients were assessed through the Hamilton Rating Scale for Depression (HAM-D). Allelic and genotypic frequencies in MD and BD subjects were compared with those of each disorder and healthy group using the χ2 statistics. Repeated measures ANOVA was used to test possible influences of SNPs on treatment efficacy.
Results
The rs9647635 A/A was more represented in subjects with BD as compared with MD and healthy subjects together. The rs9647635 A/A was also more presented in patients with MD than in healthy subjects. With regard to the allelic analysis, rs9647635 A allele was more represented in subjects with BD compared with healthy subjects, while it was not observed between patients with MD and healthy subjects.
Conclusion
Our findings provide potential evidence of an association between some variants of AHI1 and mood disorders susceptibility but not with clinical outcomes. However, we will need to do more adequately-powered and advanced association studies to draw any conclusion due to clear limitations.
INTRODUCTION
Mood disorders are severe, chronic psychiatric disorders that represent a major public health concern.1,2 Family, twin and adoption studies show evidence for a strong genetic component in mood disorders, with a relative contribution of genetic factors as high as 30-40% for major depressive disorder (MD)3 and 60-85% for bipolar disorder (BD).4
In the present study we focused on 4 single nucleotide polymorphisms (SNPs) within the Abelson helper integration site-1 gene (AHI1), which could be potentially involved in the etiology of MD and BD as well as in their treatment response.
The AHI1 gene locus, which is located on chromosome 6q23 (positive linkage with mood disorders in genome wide association study),5 encodes the protein Jouberin and is widely expressed in the brain.
The mouse orthologue of Jouberin, AHI1, binds to huntingtin-associated protein 1 (Hap1) to form a stable protein complex in the brain that is important for maintaining the level of tyrosine kinase receptor B (TrkB), which is critical for neuronal differentiation and brain development.6 Interestingly, the endogenous TrkB ligand in humans [i.e., the brain derived neurotrophic factor (BDNF)] is a survival factor for parvalbumine-positive interneurons, which have been thought to be involved in the physiopathology of mood disorders.2,7,8 Further, in the mouse brain AHI1 is abundant in the hypothalamus and amygdale,6,9 two important brain regions whose dysfunction can lead to affective disturbances.10
In a recent animal study,11 mice with neuronal AHI1 deficiency show reduced TrkB level in the brain and depressive phenotypes, which can be alleviated by antidepressant drugs or by overexpression of TrkB in the amygdala. It can be possibly assumed that overexpressed TrkB in the mouse amygdala could ameliorate the depressive phenotype of AHI1 mutant mice since the TrkB signaling plays a critical role in the depression associated with neuropsychiatric disorders.11,12,13 In addition, the loss of human AHI1 or mouse AHI1 in distinct types of cells seems to influence different cellular signaling pathways or function, which is also crucial pathway to be involved in the development and treatment response in mood disorders.14,15 Recently, animal research was taken to investigate the mechanism whereby alteration in AHI1 expression may be implicated in the pathogenesis of neuropsychiatric disorders. Lotan et al.16 conducted AHI1 heterozygous knockout (AHI1+/-) mice study to see the relationship between AHI1 gene and stress resilience, where they evidenced that under-expression of the AHI1 gene during neurodevelopment brings about relative resilience to various stressors during adulthood.16 Such resilience has been manifested by an anxiolytic-like behavioral phenotype, accompanied by a blunted response of the autonomic nervous system and the hypothalamus-pituitary-adrenal axis (HPA axis).16 That study proposes a potential link of AHI1 under-expression with a defect in the process of threat detection.16
Based on aforementioned, the present study aimed to investigate whether a set of SNPs within the AHI1 gene could be associated with MD and BD, and to explore whether such variants could be involved in clinical outcomes in patients with MD and BD.
METHODS
Subjects
The sample under investigation in the present study comprised 184 in-patients with MD and 170 in-patients with bipolar disorder (BD). All patients are native Koreans and were consecutively recruited at the Department of Psychiatry of The Catholic University of Korea. Patients were eligible for inclusion if they had a documented clinical diagnosis of MD and BD according to the DSM-IV criteria, as assessed by the Mini-International Neuropsychiatric Interview (MINI).17 The treatments were based on naturalistic treatment settings without any particular restrictions (i.e., type of pharmacological treatments and presence of concomitant co-morbidities). However, patients were excluded if they currently had severe or unstable medical and neurological conditions, were being treated with a long-acting antipsychotic, and had concomitant alcohol and substance abuse disorders. The choice not to adopt restrictive inclusion and exclusion criteria was motivated by the decision to include a sample of subjects that could be representative of the usual psychiatric inpatients in Korea. A further sample of 170 Korean psychiatrically healthy subjects, who underwent the same assessment as psychiatric patients to exclude possible psychiatric disorders and who were asked for the presence of any known psychiatric disorder in first and second degree relatives, and came from the same location as the psychiatric patients in the present study, was also included to compare genotype and allelic frequencies among three groups under investigation. All MD and BD patients admitted to the hospital were assessed for the severity of illness both at baseline and discharge by the administration of psychometric rating scales. In particular, MD severity was assessed by the administration of the Hamilton Depression Rating Scale-17 items (HAM-D).18 Raters were trained with the specific instruments with good inter-rater reliability (k>0.8). On the other hand, no psychometric evaluation was available for BD patients. Additionally, the following clinical and demographic variables were recorded for all the patients recruited: gender, age, age at onset, familiar history of psychiatric disorders, lifetime suicide attempts, duration of admission, medications at discharge and concomitant anxiolytics. The study protocol was approved by the institutional review board (approval number HC10TISI0031).
Statistical analyses
Statistical analyses were performed using "R cran" environment (http://cran.r-project.org/). The main outcome measures of the present study were: 1) differences between genotypic and allelic frequencies in patients with BD and MD compared with each group, 2) possible influence of the 4 SNPs under investigation on clinical improvement as measured by HAM-D in MD patients.
Differences in the allelic and genetic frequencies between healthy subjects and patients were calculated using the χ2 statistics. Repeated measures ANOVA was used to investigate the association among genotypes and the variation over time of HAM-D scores in MD patients. In the case of positive findings, the following clinical variables were added as covariates in order to investigate possible confounders: gender, age, age at onset, familiar history of psychiatric disorders, lifetime suicide attempts, duration of admission, medications at discharge and concomitant anxiolytics.
Haploview 3.2 was used to generate a linkage disequilibrium (LD) map and to test for Hardy-Weinberg equilibrium (HWE).19 Tests for associations using multi-marker haplotypes were performed using the statistics environment "R" package "haplo.score". Gender, age, age at onset, familiar history of psychiatric disorders, lifetime suicide attempts, duration of admission, medications at discharge and concomitant anxiolytics were added as covariates in the case of positive findings. Permutations (n=10.000) were performed to estimate the global significance of the results for all haplotype analysis. Only haplotypes with >1% prevalence were included in the analysis. All p-values were 2-tailed. In order to reduce the likelihood of false positive findings, statistical significance was set at the level of 0.01, as calculated by means of the false discovery rate (FDR) which allows for a correction of multiple testing without being as conservative as the Bonferroni correction.20
G Power (http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/) was employed to perform the power analysis. With these parameters (p=0.01) we had a sufficient power (0.80) to detect an effect size of 0.2, that as an example, corresponded to an odds ratio of 1.9 between the 3 groups of patients and the group of controls and to detect small-medium effect sizes (d=0.3 for each diagnosis) for patients with BD and MD, carrying the CC genotype of rs11154801 as compared with those carrying the AC genotype.
SNP selection
SNP relative information was retrieved by the Database of SNPs. A total of four SNPs (rs11154801, rs7750586, rs9647635, rs9321501) for AHI1 were selected to be investigated. The main criteria for this selection concern the report of their genetic frequency, their role in translation, their potential functional significance as well as bibliographic references were strongly taken under consideration.
Genotyping
Genomic DNA was extracted from blood by standard methods and quantified. High-throughput genotyping using a pyrosequencer (Biotage AB, Sweden) was used for genotyping the four SNPs (rs11154801, rs7750586, rs9647635, rs9321501) of AHI1 under investigation. PCR primers (Bioneer, Daejeon, Korea) and sequencing primers (Bioneer) used for the pyrosequencing assay were designed by using the Pyrosequencing Assay Design Software v.1 (Biotage), and 1 primer of each primer set was biotinylated. Two independent investigators blind to the clinical status of samples checked all genotypes independently. Samples showing ambiguous alleles were discarded if they showed the same features on repeated genotyping. The final call rate was more than 99.6% for each SNP.
RESULTS
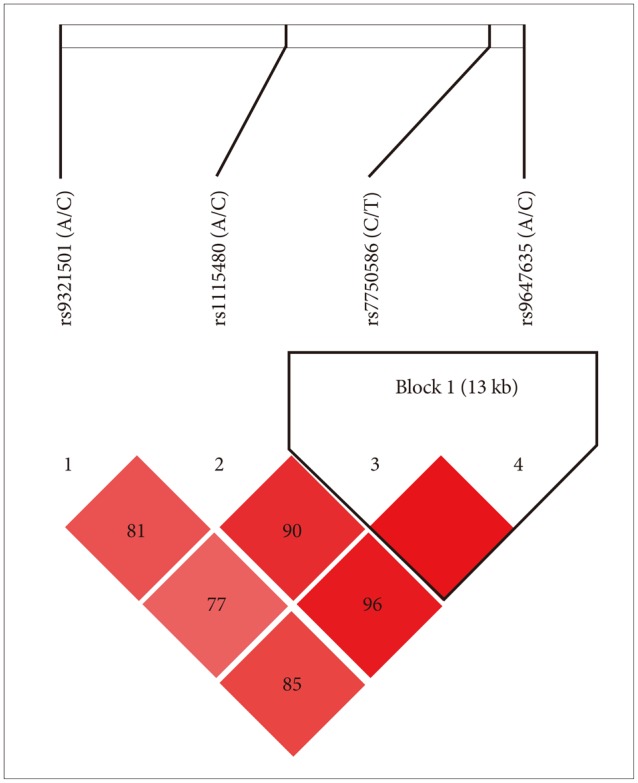
Socio-demographic features of the samples such as gender, age and further clinical and socio-demographical variables are reported in Table 1. For control subjects, only data on gender and age were collected. Patients and controls did not differ with regard to gender and age. All the considered SNPs were in HWE in the whole sample (Table 2). Strong linkage disequilibrium was observed between all SNPs, particularly between rs7750586 and rs9647635, rs11154801 and rs9647635, rs11154801 and rs7750586 (Figure 1).
Differences between Genotype and Allele Frequencies in MD and BD Patients and Healthy Controls
There were significant differences in the genotype frequencies of rs9647635 across different groups of subjects (Table 3). The rs9647635 A/A was more represented in subjects with BD as compared with patients with MD (χ2=20.345; p<0.001) and healthy subjects (χ2=34.537; p=0.008). In addition, the rs9647635 A/A was also more presented in patients with MD than in healthy subjects (χ2=9.723; p=0.008). These results were partially confirmed in the allelic analysis: The rs9647635 A allele was more represented in subjects with BD (Table 3).
However, there were no further significant differences between genotype and allele frequencies in patients with MD, BD and in healthy controls. Further, there were no differences in haplotype frequencies across samples.
AHI1 variants and clinical improvement in MD subjects
The haplotype analysis did not reveal any significant association with clinical improvement. No further genotype, allele and haplotype under investigation were significantly associated with improvement on HAM-D total scores.
DISCUSSION
The present study aimed to investigate whether 4 SNPs within the AHI1 gene could be associated with MD and BD, and whether the same variants could predict clinical outcomes in MD patients treated with antidepressants.
To the best of our knowledge, this is the first study investigating a relationship between AHI1 and mood disorders. Our results suggest a possible role of rs9647635 on BD and MD susceptibility: a significant higher proportion of subjects with BD carried the A/A genotype and the A allele when compared with other groups. Although such significant genotype difference of rs9647635 was also seen between patients with MD and healthy subjects, other SNP markers failed completely to do so. Despite scarce information about AHI1 gene for mood disorders, our exploratory results suggest that rs 9647635 should be possibly involved in the development of MD and BD, respectively, although such variants were not associated with the treatment response in patients with MD.
Considering that AHI1's extensive developmental role, from essential cellular signaling organelles, such as the primary cilium, to neuronal networks and complex organ systems, it should not be excessive if we think that Ahi1 gene should be a promising candidate gene for neuropsychiatric disorders including mood disorders.16 In fact, a number of research groups have shown significant association of the AHI1 gene with schizophrenia21 and autism spectrum disorders,22 which are replicated in a large-scale association studies.23,24 Furthermore, according to the recent AHI1 expression study in patients with BD and schizophrenia,25 the patients with early age of onset showed higher AHI1 expression than controls and later onset patients with schizophrenia. In such study, although no difference in brain expression of AHI1 in schizophrenia or BD patients compared to controls was found, there was a genotypic difference in AHI1 expression for SNP rs9321501. Genotypes that included the under-transmitted C allele (CC/AC) showed lower expression than the homozygous AA genotype. It might be possibly interpreted that the higher expression in AHI1 mRNA in early-onset and late-onset schizophrenia patients may be a putative endophenotypes of schi-zophrenia, such as neurocognitive performance.
According to the recent animal model,16 the under-expression of the Ahi1 gene during neurodevelopment was found to cause a relative resilience to various stressors during adulthood. Such resilience was exerted by an anxiolytic-like behavioral phenotype, accompanied by a blunted response of the autonomic nervous system and the HPA axis. Another animal model11 also proposed that neuronal AHI1 deficiency may cause a reduced TrkB level in the brain and should bring about depressive phenotypes, which can be alleviated by antidepressant drugs or by overexpression of TrkB in the amygdala. AHI1 deficiency also prompts the degradation of endocytic-TrkB and reduces TrkB signaling in neuronal cells, supporting that increased degradation of TrkB can induce depression and that such impaired pathway may serve as one of putative therapeutic targets for depression.11 Hence, our preliminary findings may add valuable human genetic data for the investigation of the role of AHI1 gene in the development and treatment response for mood disorders in combination with those form previous innovative animal model studies.
However, there are many limitations of the present work. Unfortunately, it was not possible to investigate the role of rs9647635 in response to mood stabilizers due to the lack of psychometric assessment in bipolar patients. Candidate gene studies, such as the present one, are associated with a high likelihood of false positive findings.26 Hence, further replications in independent samples are needed to confirm our results. A further concern is related to the use of several drugs with different mechanisms of action for each cohort of patients that do not allow one to draw definitive conclusions with regard to the influence of the SNPs under investigation upon specific drugs or classes of drugs. However, our decision to include patients treated with different drugs could have the advantage of being closer to "real world" clinical practice. Additionally, our categorization of psychiatric disorder was based on current DSM-IV criteria, as assessed by the MINI. However, with this instrument there is neither the ability to rule out the possibility that patients with MD could switch in the future to a diagnosis of BD, nor to exclude a possible switch to schizoaffective disorder. Such issues are important for genetic studies, as shown by recent findings suggesting that BD and schizophrenia could share many important risk genes.27 Furthermore, we asked healthy controls to report only known psychiatric disorders among first and second-degree relatives, thus limiting the possibility to detect whether sub-threshold or untreated psychiatric disorders among family members of healthy control subjects could exist. A further possible limitation of the present study could be imputed to the incomplete coverage of genes under investigation. Also, we did not control for population heterogeneity, though all subjects belonged to Korean ethnicity, which is considered to be genetically homogenous.28 Finally the small sample size does not allow our data to have generalizability and thus we have to in-crease the sample power in future studies.
In conclusion, our study preliminary suggests a possible role of AHI1 variants on mood disorders susceptibility, with rs9647635 being the most promising candidate polymorphism. However, further research is needed to confirm and extend our results, in particular future studies will need to include a higher number of SNPs covering larger portions of genes and more homogeneous groups of drugs.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C0003).