The Mediating Effect of Psychological Distress on the Association between BDNF, 5-HTTLPR, and Tinnitus Severity
Article information
Abstract
Objective
To investigate the association between genetic polymorphisms of brain-derived neurotrophic factor (BDNF) or serotonin transporter gene-linked polymorphic region (5-HTTLPR) and tinnitus, and the mediating effects of psychological distress on this association.
Methods
Eighty-six patients experiencing tinnitus and 252 controls were recruited. The Tinnitus Handicap Inventory was used to assess the severity of tinnitus and the Beck Depression Inventory-II (BDI-II), Beck Anxiety Inventory-II (BAI-II), and the Korean version of the Brief Encounter Psychosocial Instrument (BEPSI-K) were used to assess psychological distress. We compared the association of BDNF rs6265 (Val66Met) and 5-HTTLPR variants in the two groups. The mediating effects of BDI-II, BAI-II, and BEPSI-K were examined using multiple regression analysis and validated by the Sobel test and bootstrapping.
Results
No significant differences were found between the groups regarding BDNF Val66Met and 5-HTTLPR, but the 5-HTTLPR variants trended toward association. Depressive symptoms appeared to act as a mediator on the relationship within the 5-HTTLPR s/s genotype and the severity of tinnitus.
Conclusion
Our findings provide a speculative idea on the association between the serotonergic system and tinnitus and suggest that depressive symptoms act as a mediator in tinnitus. Therefore, screening for depressive symptoms in patients with tinnitus is essential and intervention for depressive symptoms may help alleviate the severity of tinnitus.
INTRODUCTION
Tinnitus is defined as an awareness of sound in the ears or head in the absence of an external stimulus [1]. It is a common clinical symptom affecting up to 21% of the adult population [2]. In addition, about 1–3% of the population is extremely bothered by tinnitus and they report that tinnitus negatively affects their quality of life by causing symptoms such as difficulty concentrating or sleep disturbances [3]. Tinnitus is also a significant cause of stress [4], and, furthermore, a number of studies have demonstrated that tinnitus is associated with several psychiatric disorders [4-10]. Depression and anxiety disorders are known to be some of the most common comorbid psychiatric disorders in patients with tinnitus [11-16]. Tinnitus causes psychological distress, while alternatively, the presence of psychological distress such as depressive symptoms and anxiety may exacerbate the severity of tinnitus by reducing an individual’s tolerance of tinnitus [4]. This suggests that there may be a shared molecular basis of tinnitus and these psychological distresses.
Interestingly, with respect to the severity of tinnitus, the sounds heard by patients with severe tinnitus are not different in loudness, pitch, or quality from those heard by people who are not troubled by their tinnitus [3]. The effect of tinnitus on individuals varies, and the degree of annoyance is not directly related to the perception of tinnitus [17]. In addition, tinnitus is often accompanied by hyperacusis which defines as increased sound sensitivity or decreased sound tolerance, misophonia which means hatred or dislike of certain sounds, and phonophobia which stands for the fear of or aversion to certain sounds [17]. These clinical characteristics suggest that vulnerability to tinnitus related to psychological aspects is important in explaining why some people are more troubled by their response to sounds that others live with quite comfortably [3].
Individual susceptibility to tinnitus is formed by the interaction of genetic and environmental factors [17]. However, the genetic risks of tinnitus – rather than environmental ones – remain largely unknown and the effects of psychological distress on the association between tinnitus and genetic factors have not been further identified.
In the past decade, several studies have been performed to identify the specific genetic factors in tinnitus. Twin-based epidemiological studies have estimated a moderate heritability for tinnitus and found a greater concordance rate in monozygotic twins compared to dizygotic twins [18,19]. Several case-control association studies have been performed with hypothesis-driven candidate genes, including a serotonin receptor and transporter (HTR1A, SLC6A4) [20,21], genes involved in potassium recycling pathway (KCNE1, SLC12A2) [22,23], and neurotrophic factors (BDNF, GDNF) [24,25]. Of these, genes encoding neurotrophic factors are likely candidates [17]. Neurotrophic factors play key roles in tonotopic organization of the central auditory pathway [17]. In particular, brain-derived neurotrophic factor (BDNF) plays a key role in the early development of the central auditory pathway and the inner ear sensory epithelium [25]. Also, BDNF protects the inner ear from trauma and BDNF expression patterns respond dynamically in the central and peripheral auditory systems following traumatic acoustic stimuli [26]. Tinnitus may be conceptualized as a condition of loss of inhibition and hyperexcitability in auditory neural networks that is often precipitated and maintained by stressful environments, but it can also induce regeneration processes and resolve under favorable circumstances [27,28]. In animal models, tinnitus is accompanied by peripheral signs that regeneration is in progress [29] and these responses to tinnitus correlate with the expression of BDNF [30]. Furthermore, previous research has found that serum BDNF levels were lower in patients with tinnitus than controls [31] and a negative correlation was seen between plasma BDNF levels and symptom severity in patients with tinnitus [32]. A single nucleotide polymorphism in BDNF (rs6265), a transition of guanine to adenine occurs in the 196th nucleotide, results in an amino acid substitution from valine to methionine at codon 66 (Val66Met) in the prodomain of the BDNF [33]. BDNF Val66Met was reported as a functional missense substitution and to be associated with tinnitus severity [25]. In addition, previous studies have suggested that the BDNF Val66Met polymorphism could alter stress vulnerability [34] and may increase susceptibility to depressive symptoms [35].
Serotonin is another candidate molecule because serotonergic fibers and terminal endings are present in most auditory nuclei in the central nervous system, the inferior colliculus, the nuclei of the lateral lemniscus, the superior olivary complex, and the cochlear nucleus [36]. Synaptic serotonin is regulated by the serotonin transporter, which plays a pivotal role in brain serotonin homeostasis [36]. The 5-HTTLPR is a polymorphic region on the serotonin transporter gene including a 44-bp insertion-deletion in the promotor region, resulting in either a long (l) or short (s) allele, which is a functional polymorphism [20]. The s allele has been reported to be associated with lower transcriptional activity compared to the l allele variant [37]. A casecontrol study evaluating the role of serotonin transporter gene in tinnitus has shown that there was a significant association between the 5-HTTLPR polymorphism and severity of tinnitus [20]. In addition, previous studies found that the s allele of 5-HTTLPR was associated with depressive symptoms38 and anxiety [39].
Based on these studies, we hypothesized that there is an association between the BDNF or 5-HTTLPR polymorphisms and tinnitus in the Korean population and that individual psychological distress can affect this association. Therefore, the aims of the present study were to investigate the association of BDNF Val66Met or 5-HTTLPR polymorphisms with tinnitus and the mediating effects of psychological distress, such as depressive symptoms, anxiety, and stress, on this association.
METHODS
Study participants
This study included 120 patients who visited the otorhinolaryngology outpatient clinic at Seoul St. Mary’s Hospital with a chief complaint of tinnitus on their first visit. After taking the patient’s history, a thorough oto-endoscopic examination and audiological evaluation including pure-tone audiometry and matching of tinnitus were performed to determine whether the patient was appropriate for the study. For the homogeneity of the study subject, patients with one or more of the following were excluded: objective tinnitus such as vascular or muscular origin tinnitus or patulous Eustachian tube, air-bone gap more than 10 dB on pure-tone audiometry, sensorineural hearing loss worse than 30 dB in the frequency range of 250 to 6,000 Hz on pure-tone audiometry, middle or external ear problems, Meniere’s disease, otosclerosis, vestibular schwannoma, history of previous neurotologic surgery, history of taking ototoxic drugs, history of temporal bone trauma, other uncontrolled medical and neurological diseases, and a current history of a psychiatric disorder such as depression. Of the 120 patients initially screened, 27 with hearing loss, four with current depression, and one with both hearing loss and current depression were excluded. Two of the patients who were left out were also excluded because blood samples for genetic analyses of BDNF Val66Met and 5-HTTLPR were not collected based on concerns regarding blood draw and genetic analysis, so 86 patients were finally included in the analysis.
For the control group, 252 individuals who visited the same hospital for medical checkups and had no tinnitus, no other uncontrolled medical or neurological diseases, and had not been diagnosed with a psychiatric disease were included.
All participants were informed about the study and all provided informed consent. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary’s Hospital (IRB approval number: KC16TISI0502).
Measures and procedure
To evaluate the characteristics of tinnitus, psychoacoustic parameters of tinnitus including duration, subjective site and loudness, audiological pitch, and the Tinnitus Handicap Inventory (THI) were assessed. For the site of tinnitus, patients were asked if tinnitus was subjectively felt on the left, right, bilateral, or head side. Patients were also asked about their subjective level of tinnitus loudness using a visual analog scale (VAS) ranging from 0 to 10. The audiometric measurement of tinnitus pitch was determined by asking patients to select the tone with a frequency more similar to that of the tinnitus based on the examined frequencies with different tones proposed over headphones (0.125, 0.25, 0.5, 1, 2, 4, or 8 kHz) over four categories (Low, Middle, High, Mixed). The THI was used to evaluate the perceived severity of tinnitus and its impact on life through a self-reported questionnaire with 25 items [40]. Each item had to be answered with either “yes” (4 points), “sometimes” (2 points), or “no” (0 points). This led to a total score ranging from 0–100, where a score of 0–16 indicates no handicap, 18–36 indicates mild handicap, 38–56 indicates moderate handicap, and a score of 78–100 indicates severe handicap [41]. The original version of the THI has a good internal consistency (Cronbach’s alpha=0.93) [40], as does the Korean version (Cronbach’s alpha=0.95) [42].
Patients with tinnitus were also assessed according to severity of depressive symptoms, anxiety, and stress using the Beck Depression Inventory-II (BDI-II), the Beck Anxiety Inventory-II (BAI-II), and the Korean version of Brief Encounter Psychosocial Instrument (BEPSI-K), respectively. The BEPSI-K is a 5-item self-administered instrument for measuring stress with each item rated on a scale of 1 to 5 [43]. Participants were asked to indicate the severity of the stress they had experienced in the past month. The total scores on the BEPSI-K range from 5 to 15 with high scores indicating higher stress. We used the total score in the analysis. The instrument was adapted into Korean and validated, with a good internal consistency (Cronbach’s alpha=0.80) [44].
Peripheral blood samples were collected in tubes containing ethylenediamine tetra-acetic acid and were stored at -20°C until DNA isolation. Genomic DNA was extracted from blood samples using a WizardTM Genomic DNA purification kit (Promega Madison, USA). Polymorphisms for BDNF Val66Met and 5-HTTLPR were ascertained by genotyping using polymerase chain reaction (PCR). PCR was performed twice for all samples. In the control group, five and four subjects were excluded from the genetic analysis for BDNF Val66Met and 5-HTTLPR polymorphisms, respectively, due to a lack of blood samples.
Genetic analysis
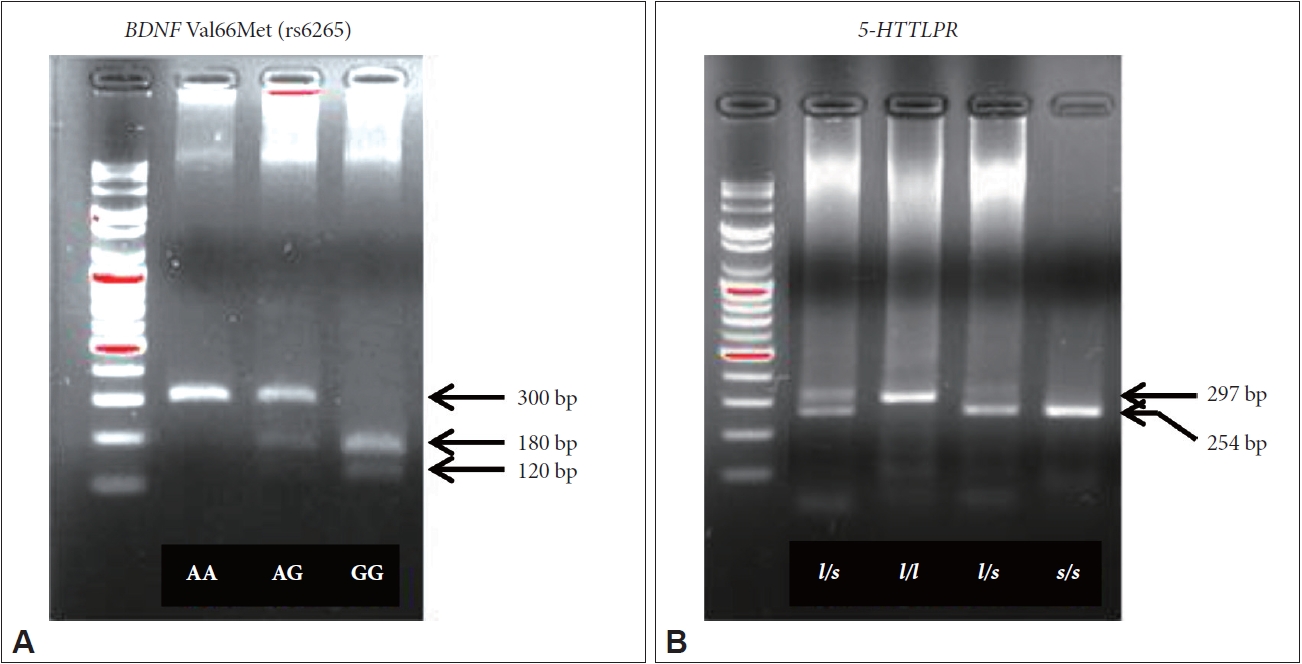
A 300-bp segment including the BDNF Val66Met polymorphism was amplified using the forward primer 5'-ATC CGA GGA CAA GGT GGC-3' and the reverse primer 5'-CCT CAT GGA CAT GTT TGC AG-3'. PCR was performed in a total volume of 14 μL containing 60 ng of genomic DNA, 1U iMaxTM II polymerase (iNtRON Biotechnology, Gyeonggi-do, Republic of Korea), 1×PCR buffer (iNtRON Biotechnology), 250 μM each of deoxyribonucleotide triphosphate (dNTPs) (iNtRON Biotechnology), and 0.2 μM each of primer pair. The PCR amplification cycle consisted of 94°C for 10 min, followed by 42 cycles of 94°C for 40 s, 60°C for 40 s, and 72°C for 1 min before a final extension step at 72°C for 5 min. 7 μL of the PCR product was incubated and digested with 10U of Pml I restriction enzyme (New England Biolabs, MA, UK) and 1X NEB buffer (New England Biolabs) at 37°C overnight. Digested fragments were analyzed by 2.5% (w/v) agarose gel electrophoresis in 0.5X TAE buffer. The genotype was determined by the size and distribution of three bands: 300-bp for the A variants (Val/Val homozygote), 180-bp and 120-bp bands for the G variants (Met/Met homozygote), and three bands for both A and G variants (Val/Met heterozygote) (Figure 1A).
A 297-bp or 254-bp segment including the 5-HTTLPR polymorphism was amplified using the forward primer 5'-CAA CTC CCT GTA CCC CTC CT-3' and the reverse primer 5'-GTG CAA GGA GAA TGC TGG AG-3'. PCR amplification was performed in a total volume of 10 μL containing 50 ng of genomic DNA, 1U AccuprimeTM Taq polymerase (Thermo Fisher Scientific, MA, USA), 1× Accuprime Buffer II (Thermo Fisher Scientific), 250 μM of each dNTP (iNtRON Biotechnology), and 0.2 μM of each primer pair. The PCR amplification cycle consisted of 94°C for 10 min, followed by 40 cycles of 94°C for 40 s, 62°C for 40 s and 72°C for 1 min before a final extension step at 72°C for 5 min. PCR products were separated in a 2.5% (w/v) agarose gel and genotypes were determined as follows: 297-bp fragment for the l/l homozygote, 254-bp fragment for the s/s homozygote, and both fragments for the l/s heterozygote (Figure 1B).
Statistical analyses
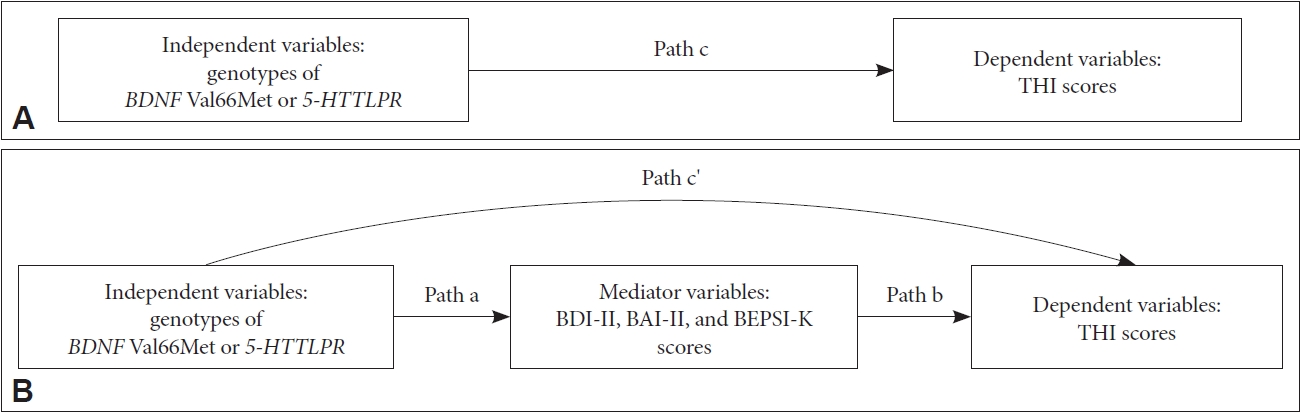
The chi-squared test was performed to check Hardy-Weinberg genetic equilibrium and analyze the BDNF Val66Met and 5-HTTLPR polymorphisms between patients and controls. Differences in demographic data and clinical variables according to genotypes in patients with tinnitus were tested using the independent-t, Mann-Whitney U, chi-squared, or Fisher’s exact test. The mediating effects based on BDI-II, BAI-II, and BEPSI-K scores were examined using multiple regressions. Baron and Kenny have proposed the following three steps in establishing mediation [45]. In Step 1, the dependent variable (THI scores in the present study) was regressed on independent variables (genotypes of BDNF Val66Met or 5-HTTLPR in the present study) to establish path “c” (Figure 2A). In Step 2, mediators (BDI-II, BAI-II, and BEPSI-K scores in the present study) were regressed on the independent variables to establish path “a” (Figure 2B) in the mediational chain. In Step 3, the dependent variable was regressed on both independent variables and mediators. This provided a test of whether mediators were related to the dependent variable (path “b”) and an estimate of the relation between independent variables and the dependent variable controlling for mediators (path “c'”). To demonstrate the mediating effect in this model, the strength of the relation between the independent variables and the dependent variable should either be eliminated (complete mediators) or significantly decreased (partial mediators).
After these steps were performed, the Sobel test and bootstrapping were conducted to validate the significance of the mediation effects [46,47]. The Sobel test uses standard errors, where the mediating effect is divided by its standard error to yield a Z-score of the mediating effect. If the Z-score is greater than 1.96, the effect is significant at the 0.05 level [48]. Bootstrapping is another method to demonstrate the significance of the mediation effects by calculating 95% confidence intervals (CIs) around the estimated effect; the effect is considered significant if the CIs do not include zero [47].
Statistical analyses were conducted using SPSS 24.0 (IBM Corp., Armonk, NY, USA) and the significance level was set as p=0.05 (two–tailed) in all data analyzed. Additionally, the Sobel test calculator and AMOS 21.0 (IBM Corp., Armonk, NY, USA) were used to calculate Z-scores and for the bootstrapping analysis, respectively.
RESULTS
The tinnitus group included 41 males (47.7%) and 45 females (52.3%). The age range was between 22 and 83 years and the mean age was 53.5±13.7 years. The control group included 132 males (51.2%) and 120 females (48.8%). The age range was between 17 and 80 years and the mean age was 53.1±10.4 years. There was no significant difference with regard to sex ratio and mean age between patients and control groups.
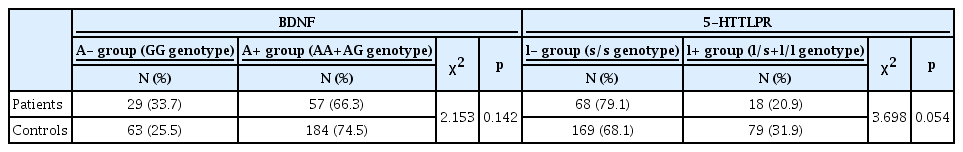
For BDNF genotypes, 66.3% (n=57) of the patients were classified into the A+ group with the A allele (AA or AG genotype) and 33.7% (n=29) were classified into the A- group without the A allele (GG genotype). In controls, 74.5% (n=184) were classified into the A+ group and 25.5% (n=63) were classified into the A- group. There was no significant difference between patients and controls (p=0.142) with regard to BDNF Val66Met polymorphisms. For the 5-HTTLPR, 20.9% (n=18) of patients were classified into the l+ group with the l allele (l/s or l/l genotype) and 79.1% (n=68) were classified into the l- group without the l allele (s/s genotype). In controls, 31.9% (n=79) were classified into the l+ group and 68.1% (n=169) were classified into the l- group. There was a trend toward significance (p=0.054) with regard to 5-HTTLPR polymorphism (Table 1). The population was in Hardy-Weinberg equilibrium.
Group differences and mediating effects of psychological distress in patients were analyzed focusing on the genotypes of 5-HTTLPR that showed a trend toward significance. There was no statistically significant difference in mean age, sex ratio, psychoacoustic parameters of tinnitus (duration, site, pitch, and loudness VAS scores), and BAI-II and BEPSI-K scores between the l+ and l- patient groups. However, the mean THI and BDIII scores were significantly higher in patients with the s/s genotype than in those with l/s or l/l genotype (Table 2).

Comparison of demographic data and clinical variables according to the genotypes of 5-HTTLPR in tinnitus patients
The results of the three steps conducted to examine the mediating effects of psychological distress on the 5-HTTLPR s/s genotype and the severity of tinnitus are as follows. In Step 1, in terms of depressive symptoms, THI was regressed on the 5-HTTLPR s/s genotype (path “c”: s/s genotype → THI) and this relationship was significant (β=0.183, p<0.05). In Step 2, depressive symptoms were regressed on the 5-HTTLPR s/s genotype (path “a”: s/s genotype → BDI-II) and this result indicated that there was a significant relationship between the 5-HTTLPR s/s genotype and BDI-II (β=0.207, p<0.05). In Step 3, we examined the effect on THI by adding the 5-HTTLPR s/s genotype and BDI-II simultaneously and found that BDIII had a significant effect (path “b”: BDI-II → THI) (β=0.366, p<0.001) and the previous relationship between the 5-HTTLPR s/s genotype and THI lost significance (β=0.107, p>0.05). Additionally, the effect of the 5-HTTLPR s/s genotype on THI can be implicated as a reduction of regression coefficients in Step 3 (β=0.107) from Step 1 (β=0.183), which means BDI-II mediates 5-HTTLPR s/s genotype and THI (Table 3). The Sobel test and bootstrapping were performed to demonstrate the statistical significance of the mediating effects of BDI-II. The Z score was calculated to be 2.032 in the Sobel test and the 95% CIs were 0.023–0.144 in the present study. Therefore, BDI-II was a statistically significant mediator.

Results of multiple regressions to identify the mediating effect of BDI-II on the association between the genotype of 5-HTTLPR and THI
However, in terms of anxiety and stress, the relationships between BAI-II or BEPSI-K and the 5-HTTLPR s/s genotype were not significant in Step 2 (path “a”: s/s genotype → BAI-II or BEPSI-K), so the mediating effects of BAI-II and BEPSI-K were not identified.
DISCUSSION
In the present study, we have investigated the association between individual BDNF Val66Met or 5-HTTLPR variants and tinnitus severity, and the mediating effects of depressive symptoms, anxiety, or stress on the association. While no significant difference was found between genotypes of patients and controls regarding BDNF Val66Met and 5-HTTLPR, the 5-HTTLPR variant trended toward association. Interestingly, depressive symptoms showed a mediating effect in the relationship between the 5-HTTLPR s/s genotype and severity of tinnitus.
In the past decade, several studies to identify specific genetic factors for tinnitus have been performed, but the field is still in its infancy. Nevertheless, prior genetic studies support that genes encoding neurotrophic factors may give promising results that warrant further study [17]. Sand et al. [25] have shown that five glial cell-derived neurotrophic factor (GDNF) and BDNF variants account for 16% of variance in tinnitus severity. GDNF is essential for the maintenance of neurons and has been shown to enhance re-growth of adult neurons following neural insult, especially in dopaminergic and motor neurons [49-51]. Previous studies suggested that adaptation to tinnitus is dependent on the process of neuroplasticity and on the restoration of dysfunctional auditory pathway [52-54]. Hence, it is assumed that the role of glial cells in the restoration of auditory function [55] and the preventive effects of BDNF on damage to the auditory pathway by tinnitus [56,57] are related to the association between genes encoding neurotrophic factors and tinnitus. However, the present study was unable to demonstrate an association between BDNF Val66Met and tinnitus. This inconsistent result is likely to be related to differences in the inclusion criteria across studies. In a previous study [25], patients with tinnitus accompanied by hearing loss were also included, and more men were recruited than women (men:women, approximately 2:1) compared to the present study. Even though little is known about the genetic contribution of sex and comorbidities like hearing loss to tinnitus, there is a tendency for men to be more affected by tinnitus than women [58,59] and some cases of tinnitus are linked to hearing loss [60].
Although no significant difference was identified between 5-HTTLPR genotypes in patients and controls, the 5-HTTLPR s/s genotype showed a trend of association with tinnitus and patients with this genotype had a higher severity of tinnitus. Some preliminary studies suggested that serotonergic activity and modifications of serotonergic neurotransmission contribute to the generation or perception of tinnitus [36]. Serotonergic neurons are present in the auditory pathway [61] and serotonin modulates the response of inferior colliculus neurons to auditory stimuli by regulating neural firing activity and frequency tuning [62]. These findings suggest that dysfunction in serotonergic activity could inhibit the modulation of neural networks in the auditory pathway and contribute to tinnitus [36]. In addition, Deniz et al. [20] reported that patients with tinnitus with the 5-HTTLPR l/l genotype had higher VAS scores of tinnitus severity, and discomfort and attention-deficit caused by tinnitus. However, in the present study, the severity of tinnitus was significantly higher in patients with the s/s genotype than in those with the l/s or l/l genotype. This rather contradictory result may partly be explained by differences in ethnicities, such as a difference in 5-HTTLPR allele frequencies between Asians and other ethnic groups, since the s allele is found in 79% of Asians and 42% of Caucasians [63-65]. In the same vein, a previous study in Koreans showed that the antidepressant response was differentially associated with the s allele of 5-HTTLPR compared to the l allele [66]. However, these associations were opposite to those reported in the Caucasian population [67,68]. These findings suggest that ethnic differences in allelic frequencies of 5-HTTLPR might be responsible for the inconsistent findings in the studies of the association between tinnitus and 5-HTTLPR across different populations similar to the present results.
It is interesting to note that the current study found that patients with the 5-HTTLPR s/s genotype had a higher severity of depressive symptoms, and depressive symptoms completely mediated the relationship between the 5-HTTLPR s/s genotype and the severity of tinnitus. These findings provide further support for the hypothesis that there is a relationship between the molecular basis of tinnitus and depressive symptoms, and serotonin seems be a potential candidate molecule. Furthermore, these results suggest that depressive symptoms are an important contributor to the severity of tinnitus and could affect differences in the severity and course of tinnitus symptoms. Similar to present results, a previous study has reported that depressive symptoms strongly predicted increased discomfort caused by tinnitus and decreased tolerance to tinnitus [5].
These findings have important implications for clinical work in that depressive symptoms should be screened preemptively in patients with tinnitus, and early intervention for depressive symptoms is essential in managing tinnitus symptoms. In addition, we can infer that antidepressants such as selective serotonin reuptake inhibitors (SSRIs), which modulate serotonergic transmission, can be effective in the treatment of tinnitus. Although prior reports showed mixed results regarding the efficacy of antidepressants, some randomized controlled trials reported that higher doses of SSRIs and tricylic antidepressants were effective for patients with tinnitus and depression, anxiety, or insomnia [3,69,70]. Among SSRIs, sertraline was found to be more effective than placebo in patients with severe refractory tinnitus [71].
The present findings should be interpreted with caution because of the following limitations. First, our sample size was modest, and subjects were recruited from the same hospital. Because of the sample size, genotypic association tests were performed with two groups (A+ and A- groups for BDNF, l+ and l- groups for 5-HTTLPR) rather than three groups (AA, AG, and GG groups for BDNF, s/s, l/s, and l/l groups for 5-HTTLPR). Second, otolaryngologic examination, audiological evaluation, and assessment of psychological distress were not performed in the control group although the history of current tinnitus and psychiatric disorders was assessed. Third, subjects with severe hearing loss and current psychiatric disorders were excluded to preserve homogeneity in individuals with tinnitus. However, a previous study has proposed that there is an association of familial forms of tinnitus with hearing loss, so tinnitus in conjunction with hearing loss may predict familial tinnitus [72]. Therefore, to validate our findings, further studies with a large number of patients with tinnitus and controls including the co-existence of hearing loss or depression are required.
Despite these limitations, the present study raises the possibility of the association of a functional polymorphism of the serotonin transporter gene with tinnitus and demonstrated that depressive symptoms have a strong mediating effect on the severity of tinnitus. These findings may help us understand inter-individual differences in the severity and course of tinnitus symptoms. Furthermore, this study implicates that screening for depressive symptoms in patients with tinnitus is necessary and early intervention such as serotonin replacement therapy through SSRIs for depressive symptoms may help to alleviate the severity of tinnitus.
Acknowledgements
This study was supported in part by grants from the Basic Science Research Program of the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2018R1D1A1A02048972) and Research Fund of Seoul St. Mary’s Hospital, The Catholic University of Korea (2018).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jung Jin Kim, Shi Nae Park, Jo-Eun Jeong. Data curation: Sekye Jeon, Shi Nae Park, Jo-Eun Jeong. Formal analysis: Jo-Eun Jeong, Eun Young Cho. Funding acquisition: Shi Nae Park. Investigation: Sekye Jeon, Shi Nae Park, Jo-Eun Jeong. Supervision: Jung Jin Kim, Shi Nae Park, Kyung Sue Hong. Writing—original draft: Jo-Eun Jeong. Writing—review & editing: Jo-Eun Jeong, Jae Sang Han, Eun Young Cho, Shi Nae Park, Jung Jin Kim.