Incongruent Expression of Brain-Derived Neurotrophic Factor and Cortisol in Schizophrenia: Results from a Randomized Controlled Trial of Laughter Intervention
Article information
Abstract
Objective
Schizophrenia has been associated with dysfunction of the hypothalamic-pituitary-adrenal axis. Furthermore, alterations in neurotrophic factors might contribute to the pathogenesis of schizophrenia. We aimed to evaluate the effects of a simulated laughter intervention on the levels of cortisol and BDNF and to determine whether the effects associated with simulated laughter could be sustained after discontinuation of the intervention.
Methods
In this randomized controlled study, patients with schizophrenia according to DSM-IV clinical criteria were randomly assigned to receive either 8-week-long simulated laughter intervention (n=32) or treatment-as-usual group (control group, n=27). The serum levels of BDNF and cortisol were measured at baseline, week 8, and four weeks after discontinuation (week 12) of the intervention program.
Results
After an 8-week simulated laughter intervention, the laughter group had significantly higher levels of BDNF; however, four weeks after discontinuation of the intervention, the levels of BDNF significantly dropped. Interestingly, the levels of cortisol did not change significantly at week 8, but they were significantly elevated at week 12. The levels of BDNF and cortisol in the control group did not change significantly between week 0 and week 8.
Conclusion
These findings suggest that the simulated laughter intervention has an early effect on neurogenesis with a significant delayed effect on stress regulation in subjects with schizophrenia.
INTRODUCTION
Laughter, a human emotional expression, is universally produced and recognized across all contexts of social interaction. Laughter is a fundamental communicative signal, promoting person-to-person bonding and even negotiating [1]. While different fields of researchers have proposed several laugh types [2], laughter in essence can be classified into two types: emotionally-driven, involuntary (i.e., spontaneous) laughter and self-induced, non-emotional, articulated (i.e., volitional or simulated) laughter [1]. In recent decades, scientific evidence supports the notion that laughter is beneficial for both physical and mental health [3,4]. Social laughter may further trigger endorphin release which provides a neurochemical mechanism contributing to the long-term maintenance of social bonds [5].
Schizophrenia is a chronic mental disorder characterized by a combination of psychotic symptoms and cognitive and motivational dysfunction [6]. When facing social stress, patients with schizophrenia may have impaired hypothalamic-pituitary-adrenal (HPA) axis activation [7]. A blunted cortisol response in schizophrenia has been associated with more severe symptoms and worse prognosis [7-9]. Several studies have used heart rate variability (HRV) to examine the imbalance of HPA axis in schizophrenia, suggesting that patients with schizophrenia had diminished HRV compared with healthy controls, such as decreased power of high frequency (HF) and low frequency (LF) [10-14]. This pattern of reduced complexity in heart rate fluctuations in schizophrenia suggests that these individuals experience difficulty in adapting their heart rate in response to environmental stimuli. In addition, patients with schizophrenia have decreased levels of brain-derived neurotrophic factor (BDNF) [15,16], a neurotrophin that regulates neuronal survival, differentiation, plasticity, and development in the central and peripheral nervous systems [17]. The reduced BDNF expression in schizophrenia may underlie the core behavioral and cognitive symptoms of this disorder [16,18,19]. Importantly, previous studies suggest that the variation of peripheral BDNF levels in schizophrenia may be associated with the efficacy of pharmacological interventions [20]. However, whether non-pharmacological interventions can bring about BDNF changes and produce a clinical response is still unknown.
Sufficient evidence shows the benefits of simulated laughter on physiological and psychological systems in healthy people [2,3]. To date, very few studies have examined such effects in people with schizophrenia. One study assessed the effects of a humor intervention on positive and negative symptoms in schizophrenia and found not only improved in clinical symptoms of psychosis but also in rehabilitative outcomes after five weeks of humor intervention [21]. Patients with schizophrenia may be the most in need of cost-efficient means to improve their social cognition and social interaction skills [22], and a laughter intervention is a non-pharmacological, easy-to-use, and inexpensive natural therapeutic modality. The aim of this study was to evaluate the effects of simulated laughter on mood regulation, self-esteem, and other physiological domains reflecting the stress response, including heart rate variability (HRV) and the peripheral levels of BDNF and cortisol. We also tested the lasting effect of a simulated laughter intervention, which has not previously been evaluated.
METHODS
Study design
We conducted a 8-week open-label randomized controlled study of Laughing Qigong Program (LQP). Subjects were randomized to either LQP group or control group. Randomization was performed according to a computer-generated schedule with a permuted-block design. The investigators did not know the block size. The person generating the randomization schedule was not involved in determining patients’ eligibility, administering treatment, or the outcome assessment. The sample size estimation for intermediate effect sizes and 95% confidence interval with continuous outcomes corresponds to approximately 60 participants. In the end, 66 participants were randomized to either the laughter group (n=34) or the comparison group (n=32).
The experimental protocol was approved by the Institutional Review Board for the Protection of Human Subjects at the Tri-Service General Hospital, National Defense Medical Center in Taipei, Taiwan (TSHGIRB No. BT100-08). Each subject was completely informed about the procedures. Between 2011 and 2012, participants who were undergoing rehabilitation in the chronic ward of the Beitou Branch of Tri-Service General Hospital were eligible to participate. The subjects were required to be fully capable of comprehending the study’s purpose, procedures, treatments, risks and possible benefits, alternative treatments, and their right to refuse to participate. All the participants provided written informed consent and were free to withdraw their participation at any time.
Study population
The inclusion criteria were as follows: 1) age between 20 and 60 years; 2) meeting the diagnostic criteria for schizophrenia according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; 3) hospitalized for more than 6 months; and 4) motivated to join the study group. Each eligible participant was randomized to either the intervention or control group.
Individuals were excluded if they had a systolic blood pressure of >140 mm Hg or a diastolic blood pressure of > 90 mm Hg; if they had a history of a significant medical disorder, such as diabetes, cardiovascular disease, liver cirrhosis, renal disease, rheumatic arthritis, endocrinopathies or cancer; if they had a history of a neurological disorder, such as epilepsy or Parkinson’s disease; if they met the criteria for substance-related disorders, including nicotine and alcohol; and if they were involved in a special physical exercise program. Betel nut chewing, cigarette smoking, and alcohol consumption were not allowed during the study.
A series of examinations were performed, including a complete physical examination, routine biochemical panel, complete blood count, urinary test, stool study, chest radiography, and electrocardiography (ECG). The height (cm) and weight (kg) of each participant were measured using a standard balance beam scale, and the body mass index (BMI) was calculated. Medical charts were systematically reviewed in order to confirm that all participants met the above-mentioned criteria.
Laughing Qigong Program
The LQP is a combination of qigong techniques and simulated laughter with a focus on the mind and body connection. The LQP groups were carried out for 40–50 minutes by two experienced, certified trainers. The detailed procedures for LQP have been previously reported [23,24] and are briefly summarized here. The LQP is composed of four stages: 1) warm-up stage (3 min), 2) RenMai and DuMai stages (4 min), 3) transformation stage (10 min), and 4) cool-down stage (5 min). All subjects were asked to complete a minimum amount of time per stage. The transformation stage consisted of two parts–the stating of emotions and simulated laughter. The stating of emotions increases awareness of emotions and current mood states and the simulated laughter transforms negative mood states. Subjects are taught to accept negative emotional states and, rather than feeling helpless or overwhelmed, actively engage in transforming them internally while in the company of others in the LQP group. The social support of other group members helps participants realize that they are not alone in feeling their negative emotions. By providing a safe context for releasing emotions, and then transforming them in a group setting, the LQP process generates a sense of empowerment.
The LQP was conducted twice a week for 8 weeks in the laughter group and participants in the control group were asked to continue their usual daily activities and did not receive any form of exercise. All the psychotropic drugs were unchanged throughout the course of the study.
Physiological measures
The laughter group was assessed at baseline (W0), week 8 (W8, the end of the 8-week LQP), and week 12 (W12, 1-month follow-up after the LQP). The control group was assessed at W0 and W8.
The physiological measures included the serum levels of BDNF and cortisol, weight, blood pressure, and HRV. After overnight fasting, peripheral venous blood samples were drawn by venipuncture in the morning between 07:30 and 08:30 hours. The blood samples were collected in vacutainer tubes (Venosafe, Terumo Europe N.V., Leuven, Belgium) without an anticoagulant. According to the manufacturer’s instructions, samples were stored at 7°C for ~30 min for deactivation of coagulation factors. The blood samples were centrifuged for 15 minutes at 1000×g and 4°C (Rotixa 50, Hettich Zentrifugen, Mühlheim, Germany). The serum was obtained and assayed immediately or aliquoted and stored at ≤-20°C. The serum BDNF levels were measured using enzyme-linked-immunosorbent (ELISA) assay (BDNF R&D Systems, Minneapolis, MN, USA) (Cat. No. DBDOO). The serum cortisol levels were measured using a commercial ELISA kit (IBL-Hamburg GmbH, Hamburg, Germany) (Cat. No. RE52061). All blood samples were run in duplicate and the average value of the replicates was taken as the sample result.
Detailed procedures of the analysis of HRV (SA-3000P HRV Analyzer, Medicore Co. Ltd., Seoul, South Korea) have been reported previously and are briefly summarized here [25,26]. The standard frequency-domain measurements were as follows: very low frequency (VLF; <0.04 Hz), LF (0.04–0.15 Hz), HF (0.15–0.40 Hz), total power (TP), the ratio of LF to HF (LF/HF), and LF plus HF. The spectral components of HRV were analyzed as absolute units through log transformed data (ln ms2). The time-domain parameters were the mean RR intervals (MRR), the mean of all normal-to-normal interbeat intervals (MNN), and the variance of the RR intervals (VAR). The time-domain parameters were measured in milliseconds (ms). All HRV analyses were performed by a trained research nurse blinded to the protocol. Individuals who showed predominantly irregular sinus rhythm or those who had sustained atrial arrhythmias, such as atrial fibrillation or >5% ectopic complexes, were excluded from further analysis.
Psychological measures
The psychological measures included the Rosenberg Self-Esteem Scale (RSES) [27] and the. Beck Depression Inventory-II (BDI-II) [28].
The RSES is a widely used self-report instrument for evaluating individual self-esteem in social science research. By measuring both positive and negative feelings about the self, the scale is believed to be unidimensional regarding the overall feelings of self-worth or self-acceptance. It is a short, easy to administer, Likert-scale questionnaire, with ten items answered on a four-point scale with responses ranging from strongly disagree to strongly agree. Higher scores on the RSE indicate higher self-esteem.
The BDI-II is a 21-question, multiple-choice, self-report inventory. It is one of the most widely used psychometric tests for quantifying levels of depression. Each of the 21 items is related to symptoms of depression such as hopelessness and irritability, feelings of guilt or of being punished, as well as physical symptoms such as fatigue, weight loss, and lack of interest in sex. On two items (16 and 18) there are seven options to indicate either an increase or decrease in appetite and sleep. Cut-off score guidelines for the BDI-II are given with the recommendation that thresholds be adjusted based on the characteristics of the sample and the purpose of the BDI-II. The cutoffs were: 8, no depression; 9–13, minimal depression; 14–19, mild depression; 20–28, moderate depression; and 29–63, severe depression. All psychological measures were performed at baseline (W0) and week 8.
Statistical analysis
Data are presented as the mean±standard deviation or as percentages. Group differences in continuous variables were analyzed using independent-sample t-tests. Pearson’s chi-squared test was used to compare the distribution of categorical variables between groups. If the marginal distribution for the categorical variables was uneven or if there was a small value (less than five) in one of the cells, the Fisher’s exact test was used. To evaluate the treatment effects of the laughter intervention compared to no intervention, we performed a two-way repeated measures analysis of variance (ANOVA) with the intervention condition as the grouping factor and pre- and post-treatment measures as the repeated measures. Treatment effects following the discontinuation of the laughter intervention were analyzed using repeated measures analysis. A Bonferroni adjustment was used to correct for multiple comparisons.
All the statistical tests were two-sided, and p-values less than 0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Demographics and clinical characteristics
Two patients in the laughter group failed to complete the LQP and five patients in the control group refused to complete the physiological and psychological measures. Data analysis consisted of 32 subjects with schizophrenia receiving LQP and 27 control subjects (Supplementary Figure 1 in the online-only Data Supplement). Table 1 shows the demographic and clinical characteristics of subjects in the laughter group and the control group. At baseline, the laughter group did not differ from the control group on the variables of age, sex ratio, education level, height, weight, or BMI. There were no significant between-group differences in levels of BDNF and cortisol. In addition, the time and frequency domain parameters of HRV in the laughter group did not differ from those in the control group.
HRV, BDNF, cortisol, and other measures
Table 2 shows the physiological and psychological measures of subjects in both groups at W0, and W8. In the laughter group, participants experienced significantly higher levels of BDNF (p<0.001) and LF (p=0.003) and significantly lower RSES (p=0.039) after the intervention, while there was no significant change in the control group for any of these factors (Figure 1). For the two-way ANOVAs, group effect refers to the laughter group versus control group and treatment effect for pre- versus post-treatment conditions. Two-way ANOVAs showed significant interaction effects for BDNF (Figure 2), but no significant differences for all other parameters.

Effects of 8-week simulated laughter program on physiological and psychological measures in laughter group
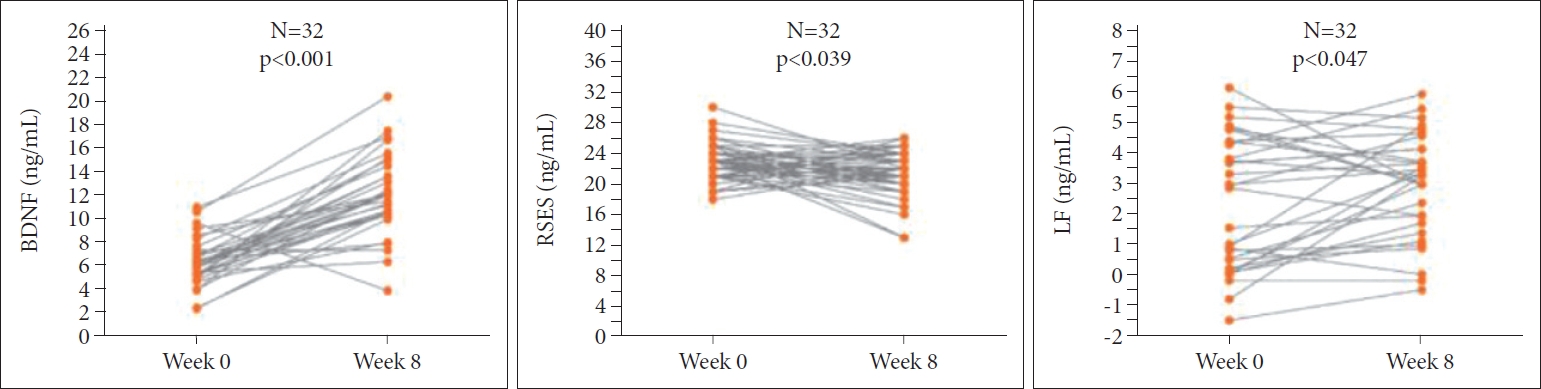
Significant difference of parameters examined by paired t-test in laughter group. BDNF: brain-derived neurotrophic factor, RSES: Rosenberg Self-Esteem Scale, LF: low frequency.
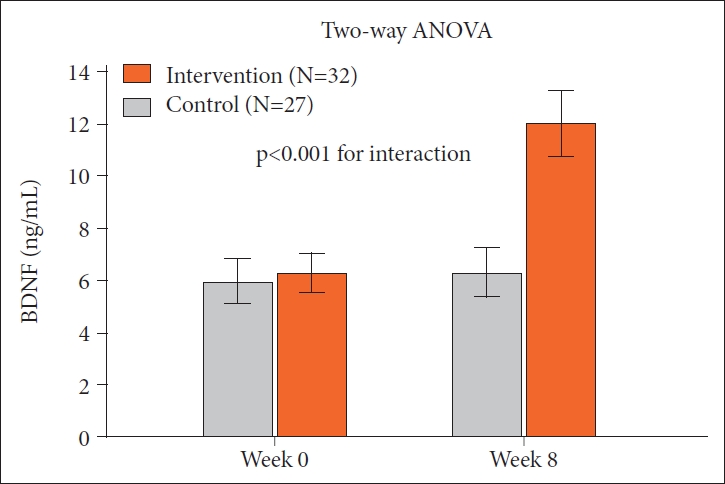
Level of BDNF before and after different treatment. P values refer to interaction effects as measured by repeated measures analysis of variance. Error bars indicate the standard deviation. BDNF: brain-derived neurotrophic factor.
Figure 3 illustrates the Bonferroni post-hoc tests for the levels of BDNF and cortisol in the laughter group. The levels of BDNF at W8 were significantly higher than those at W0 (12.1 ±3.4 vs. 6.3±2, p<0.001), and the levels of BDNF at W12 were significantly lower than those at W8 (4.9±2 vs. 12.1±3.4, p<0.001). The levels of cortisol at W8 were not significantly different from those at W0 (13.1±3.1 vs. 13±3.9, p>0.999); however, the levels of cortisol at W12 were significantly higher than those at W8 (16±3.6 vs. 13.1±3.1, p<0.001) and W0 (13±3.9 vs. 13±3.9, p=0.022).
DISCUSSION
The present study showed that an 8-week simulated laughter intervention in subjects with schizophrenia could affect the levels of BDNF, RSES, and LF of HRV. Meanwhile, this intervention did not change the levels of cortisol, other parameters of HRV, or depression state. Four weeks after discontinuation of the simulated laughter intervention, the levels of BDNF decreased but the levels of cortisol increased in the intervention group. Between-group analyses showed a significant groupby-time interaction for LF, driven by increases in LF power after the simulated laughter intervention. In contrast, the control group did not show any significant changes in these psychological and physiological measures from W0 to W8. Therefore, the cortisol response stimulated by the intervention did not occur directly during the program, but rather showed elevation after four weeks without the intervention. In line with the delayed cortisol peak, the increment of LF power is more likely to represent earlier activity of autonomic nervous system than hormonal response. As a result, although patients with schizophrenia are influenced by their negative and cognitive symptoms, the LQP might still have benefits in upregulating the levels of BDNF in the short-term and sustaining its effect on the activity of the HPA axis.
Prior evidence supports that a combination training program with resistance and aerobic exercise could significantly raise the circulating BDNF levels in patients with schizophrenia [29]. A meta-analysis indicated that acute and regular exercise has a significant impact on BDNF levels [30]. However, BDNF serum level dropped significantly four weeks after discontinuing the intervention. Therefore, whether the LQP intervention needs a minimum duration to maintain this influence needs to be clarified.
Increased complexity in heart rate fluctuations is indicative of strong cardiac adaptability, while patients with schizophrenia have been associated with cardiac autonomic dysregulation and reduced complexity of heart rate modulation [31]. Evidence indicates that the social tasks might induce intense autonomic arousal in patients with schizophrenia, with both HF and LF decreased under social stress [14]. Given the potential of compromised social functioning, such as lack of social skills and disturbances in emotional regulation, patients with schizophrenia might be refractory to available treatments [14]. It has been documented that laughter has several positive physiological effects, and one of the most important effects is to help individuals cope with stress and reduce anxiety [32]. The effect had been noticed to influence the hormone response in the pituitary, such as endorphins, and improved blood circulation, and decreased stress [33]. In brief, we found an increased LF (a composition of both parasympathetic and sympathetic activity) in the LQP group, which is an intriguing finding and has not been observed in previous studies. This suggest that LQP may be a kind of cognitive-behavioral therapy for stress coping and bring promising the impact of rehabilitative outcomes on such patients.
Previous studies have reported that physical exercise is a specific stressor that increases secretion of cortisol, a glucocorticoid hormone secreted by the adrenal cortex, in the human body [34]. The high circulating levels of cortisol signals the anterior pituitary gland to decrease adrenocorticotropic hormone (ACTH) secretion in the hypothalamic–pituitary–adrenal axis. Cortisol levels increase in proportion to the intensity of exercise, but reach an upper limit depending on the total duration of an exercise session [35]. In addition, previous studies have shown that regular exercise increases basal cortisol levels and the cortisol response is likely to be dependent on the intensity and duration of the exercise, with greater intensity and longer duration of exercise inducing a stronger cortisol response [36]. In the present study, the cortisol level at the end of the 8-week LQP was not significantly elevated in the subjects with schizophrenia. Instead, the cortisol level increased four weeks after ceasing the intervention program. One possible explanation is that restoration of a dysfunctional HPA axis might have delayed the response due to hormone production. An alternative, not mutually exclusive explanation for the delayed effect of LQP in schizophrenia is that this is a consequence of appropriate appraisal of social interaction, rather than physical effect. Albus et al. [37] discovered that patients with chronic schizophrenia have a blunted or absent cortisol response not only to physical stressors but also to psychological distress. A previous study found that blunted reactions applied only specifically to psychosocial stress but not to physical stress, and the cortisol level was negatively correlated with coping strategies in patients with schizophrenia [38]. Therefore, we suggested that LQP could improve social recognition and social interaction skills, and the secretion of cortisol might support the idea of normalization of the HPA axis in response to psychosocial stress.
It is noted that all of our subjects were prescribed psychotropic pharmacological agents such as antipsychotics, anticholinergics, benzodiazepine, lithium, valproic acid, or beta-blockers. Evidence suggests that second-generation antipsychotics, like olanzapine, quetiapine, and clozapine decrease ACTH and cortisol levels, likely through their serotonergic, adrenergic, and histaminergic pathway activities in healthy subjects [39]. The diminished cortisol response could be understood as a symptomrelated effect showing desensitization of the HPA system by recurrent ”environmental” stress. Moreover, after discontinuing the program, healthy people can continue the intervention program by themselves. However, the present group was administered the intervention program in a hospital. After discontinuation, the possibility of self-motivated LQP may be low based on the negative symptoms that are an essential feature of schizophrenia.
Limitations
This study has several limitations. First, all the participants were recruited from the chronic rehabilitation ward, which suggests that their psychiatric situation was controlled and relatively stable. Because we did not test the cognitive and clinical psychiatric symptoms of these patients, the generalizability to other stages of schizophrenia, such as the acute or remission states, might be limited. In addition, the negative symptoms of schizophrenia might confound the results and measures of impaired humor comprehension and humor appreciation during laughter. Second, we did not control the metabolic profile such as fasting glucose and total cholesterol of the subjects, which could have introduced bias. Third, we did not adjust for the category and doses of psychotropic agents, and the effects of various psychotropic agents on BDNF and cortisol might be different. Fourth, the intensity and duration of each LQP session may be too low in comparison to general aerobic exercise to have an influence. Fifth, our study only involved a small sample size; further investigations with larger sample sizes are needed.
In conclusion, although patients with schizophrenia experience psychotic and cognitive symptom-related social recognition deficits, LQP may increase their levels of BDNF and cortisol. LQP is an easy and available humor intervention for humor skill training that requires no additional equipment in a mental health setting. It provides a non-invasive and non-pharmacological method to treat mood symptoms, and it regulates stress level and provides social support. LQP can be considered an alternative and complementary treatment for rehabilitation of people with schizophrenia.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2020.0269.
Acknowledgements
This study was funded by the Civilian Administration Division of Beitou Branch, Tri-Service General Hospital, National Defense Medical Center (BAFH-101-02) and Penghu Branch, Tri-Service General Hospital, National Defense Medical Center (TSGH-PH-E 109011).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Ta-Chuan Yeh, Chih-Sung Liang. Data curation: Shu-Li Cheng, Ta-Chuan Yeh, Chih-Sung Liang. Formal analysis: Ta-Chuan Yeh, Chih-Sung Liang. Funding acquisition: Shu-Li Cheng. Investigation: Yu-Ting Tseng, Shih-Chieh Ku. Methodology: Fu-Chi Yang, HsuanTe Chu. Project administration: Hsuan-Te Chu, Chia-Kuang Tsai. Resources: Ta-Chuan Yeh, Chih-Sung Liang. Software: Chia-Kuang Tsai. Supervision: Chih-Sung Liang. Validation: Shu-Li Cheng, Fu-Chi Yang, Ta-Chuan Yeh, Chih-Sung Liang. Visualization: Shih-Chieh Ku, Yu-Ting Tseng. Writing—original draft: Shu-Li Cheng.Writing—review & editing: Ta-Chuan Yeh, Chih-Sung Liang.


