Comparison of Autonomic Reactivity to the Stress between Adjustment Disorder and Major Depressive Disorder
Article information
Abstract
Objective
Adjustment disorder (AD) remains an ambiguous diagnosis that overlaps with major depressive disorder (MDD). This study compared autonomic reactivity to the stress between AD and MDD to test for biological differences.
Methods
Physically healthy Korean male soldiers admitted to a psychiatric ward were recruited for participation. Clinical diagnoses indicated that 62 patients with AD and 47 with MDD were selected. Procedures consisted of electrocardiogram measurements according to three consecutive phases lasting five minutes each [i.e., resting, stress (including a mental arithmetic task and Stroop color word test), and recovery].
Results
The reactive trends of all heart rate variability (HRV) parameters related to the stress tasks in participants with AD did not differ from those with MDD. High-frequency HRV (a proxy of parasympathetic activity) increased during times of stress for participants with AD and MDD. Despite similar reactive trends, AD participants had higher HRV values than participants with MDD during whole phases, particularly for variables reflecting overall autonomic activity.
Conclusion
AD is associated with higher basal activity in the autonomous nervous system when compared to MDD. However, both are associated with pathophysiology indicating an altered autonomic reactivity to stress.
INTRODUCTION
Adjustment disorder (AD) is one of the most common psychiatric illnesses [1-4] and a significant risk factor for suicidal behaviors [5-8]. In particular, the prevalence rate of AD is approximately six times higher in Korean military population than in general population [9,10]. Despite its high prevalence and serious impact, research on AD is profoundly underdeveloped due to diagnostic ambiguity. It remains an unstable and subthreshold diagnosis rather than a discrete diagnostic entity. For instance, AD is frequently overlapped with other psychiatric illnesses, particularly major depressive disorder (MDD) [11]. However, longitudinal courses and therapeutic approaches indicate clear differences between AD and MDD. In contrast to MDD, AD is associated with a self-limiting course, short duration of illness, and low relapse rate [12-16]. Thus, early differential diagnoses are needed for direct service planning and enhancing therapeutic efficacy to ensure the proper management of both conditions.
The biological underpinnings of AD and MDD should be understood because differential diagnosis efforts based on symptomatology have reached their limitations [17-19]. However, the research in this area is strikingly scarce. Only two studies have tried to compare the Hypothalamic-Pituitary-Adrenal (HPA) axis dysfunction between AD and MDD [20,21], but no significant differences were found. Instead of conducting an assessment using the HPA axis, this study focused on dysfunction of the autonomous nervous system, which is another core pathophysiology of the human stress response [22]. We previously compared heart rate variability (HRV) [23] (a reliable biomarker of autonomic activity as assessed through a non-invasive measurement [24]) between AD patients and healthy controls. We found that AD patients showed reversed autonomic reactivity to the dynamic stress tasks when compared to the normal stress reaction. However, no other studies have compared the autonomic reactivity of AD to that of MDD.
This study compared autonomic reactivity between AD and MDD patients by measuring dynamic changes in HRV during stress tasks. Because AD is associated with a better prognosis, we hypothesized that the reactive patterns of HRV to stress would be less altered in AD patients compared to those in MDD patients.
METHODS
Participants
We recruited male military soldiers aged 18 to 35 who had been admitted to the psychiatric ward of the Armed Forces Capital Hospital in Seongnam, Korea. Participants diagnosed with either AD or MDD were selected for analysis in this study from August 2016 to August 2018. Experienced psychiatrists clinically diagnosed AD and MDD in participants according to the Diagnostic and Statistical Manual of Mental disorders, 5th edition (DSM-5). Participants with comorbid physical illnesses (e.g., cardiovascular diseases, hypertension, diabetes mellitus, asthma, acute respiratory infection, thyroid diseases, and/or drug intoxication) or those taking medication (e.g., sympathomimetics, anticholinergics, vasodilators, and/or anti-hypertensives) that could strongly affect the autonomic nervous system were excluded from analysis. We also excluded users of tricyclic antidepressants because of their remarkable anticholinergic and α1-adrenergic properties [25]. The study protocol was approved by the Institutional Review Board of the Armed Forces Medical Command(AFMC-12-IRB-010).
Stress task and HRV measurement
Stress tasks and related HRV measurements were performed at the time of admission by a trained medical laboratory technologist who was blinded to all clinical diagnoses. All procedures were performed in a quiet, air-conditioned room set to an ambient temperature of 22–25°C. To analyze beat-to-beat HRV, an electrocardiogram was conducted by placing a photoplethysmography sensor on the third finger of the right hand. HRV parameters were measured using the ProComp2 system and Biograph Infiniti Software (Thought Technology Ltd., Quebec, Canada). All participants were prohibited from drinking coffee, smoking, exercising, or taking any medications within a 12-hour period before the testing procedure. They were seated in a comfortable armchair and informed to relax while breathing slowly and naturally. The procedure began after a five-minute rest period. The assessment took a total of 15 minutes and consisted of the three following consecutive phases (five minutes each): Resting phase, stress phase, and recovery phase. Participants were informed to relax and stare at green spots on a nearby screen during the resting and recovery phases. Stressphase tasks consisted of a mental arithmetic task and Stroop color word test half-and-half. The mental arithmetic task consisted of a serial subtraction in increments of seven beginning with the number 1,081, while the Stroop color word test entailed that participants state the actual observed colors of written words denoting different colors (i.e., the written color did not match the displayed color). All participants were prompted to answer as fast as they could during these stress tasks.
HRV parameters
A power spectrum analysis was conducted using a nonparametric fast Fourier transformation. The distribution of spectral power was transformed into the function of frequency and then quantified into standard frequency-domain parameters [26]. The frequency-domain parameters included high frequency HRV (HF, 0.15–0.40 Hz), low frequency HRV (LF, 0.04–0.15 Hz), very low frequency HRV (VLF, 0.003–0.04 Hz), total power (TP), and the ratio of LF to HF HRV (LF/HF). As a time-domain parameter, the standard deviation of normal-to-normal interbeat interval (SDNN) was also measured.
Assessing symptom severity
All participants completed the Korean versions of the Center for Epidemiologic Studies Depression scale (CES-D) for depressive symptoms [27], Beck Anxiety Inventory (BAI) for anxiety symptoms [28], Perceived Stress Scale (PSS) for stress responses [29], and Scale for Suicide Ideation (SSI) for suicidality [30].
Statistical analysis
We compared sociodemographic and clinical characteristics between groups using Pearson’s chi-square tests for categorical variables and Student’s t-tests for continuous variables. All HRV parameters (e.g., the band powers of HF, LF, VLF, TP, and LF/HF and the SDNN) were logarithmically transformed to correct for skewed distributions. Repeated measures analyses of variance (ANOVA) were performed to analyze intergroup differences regarding changes in HRV parameters throughout the assessment, with adjustment of p values by using Bonferroni’s method (adjusted p=0.05/3). Meanwhile, intergroup differences in HRV parameters were analyzed using Student’s t-tests during each of the three phases. Finally, we performed sensitivity analyses by calculation of the Pearson’s correlation coefficients between HRV parameters and symptom severity.
All statistical analyses were performed using the SPSS 19.0 (IBM Corp., Armonk, NY, USA) statistical package.
RESULTS
Sociodemographic and clinical characteristics
Analyses were conducted on data from 62 patients with AD and 47 patients with MDD. All participants were male (mean age of 21.5±2.8 years). There was no missing data in all HRV parameters of both groups.
Table 1 shows participant sociodemographic and clinical characteristics. The AD group did not differ from MDD group regarding the possible confounders affecting HRV parameters (e.g., age, smoking, body mass index, blood pressure, and significant antidepressant use as defined by a dosage taken for more than four weeks). The mean duration of antidepressant usage was also comparable between groups (AD 5.5±7.6 weeks, MDD 6.0±9.8 weeks, p=0.823). Histories of mental illnesses, suicide attempts, and symptom severity related to depression, anxiety, stress response, and suicidality were also highly similar between groups (Table 1). The proportion of military personnel who was conscripted was higher in AD group than that of MDD group (93.5% vs. 78.7%, p=0.022).
Autonomic reactivity to mental stress
The repeated-measures ANOVAs revealed similar trends in reactive changes in HRV over whole phases between the AD and MDD groups (Figure 1). The interaction effect of group *time was not statistically significant for any HRV parameters (HF, F=0.734, p=0.481, η2p=0.007; LF, F=0.334, p=0.705, η2p=0.003; VLF, F=0.785, p=0.457, η2p=0.007; TP, F=0.458, p=0.633, η2p=0.004; LF/HF, F=0.672, p=0.512, η2p=0.006; SDNN, F= 0.656, p=0.520, η2p=0.006).
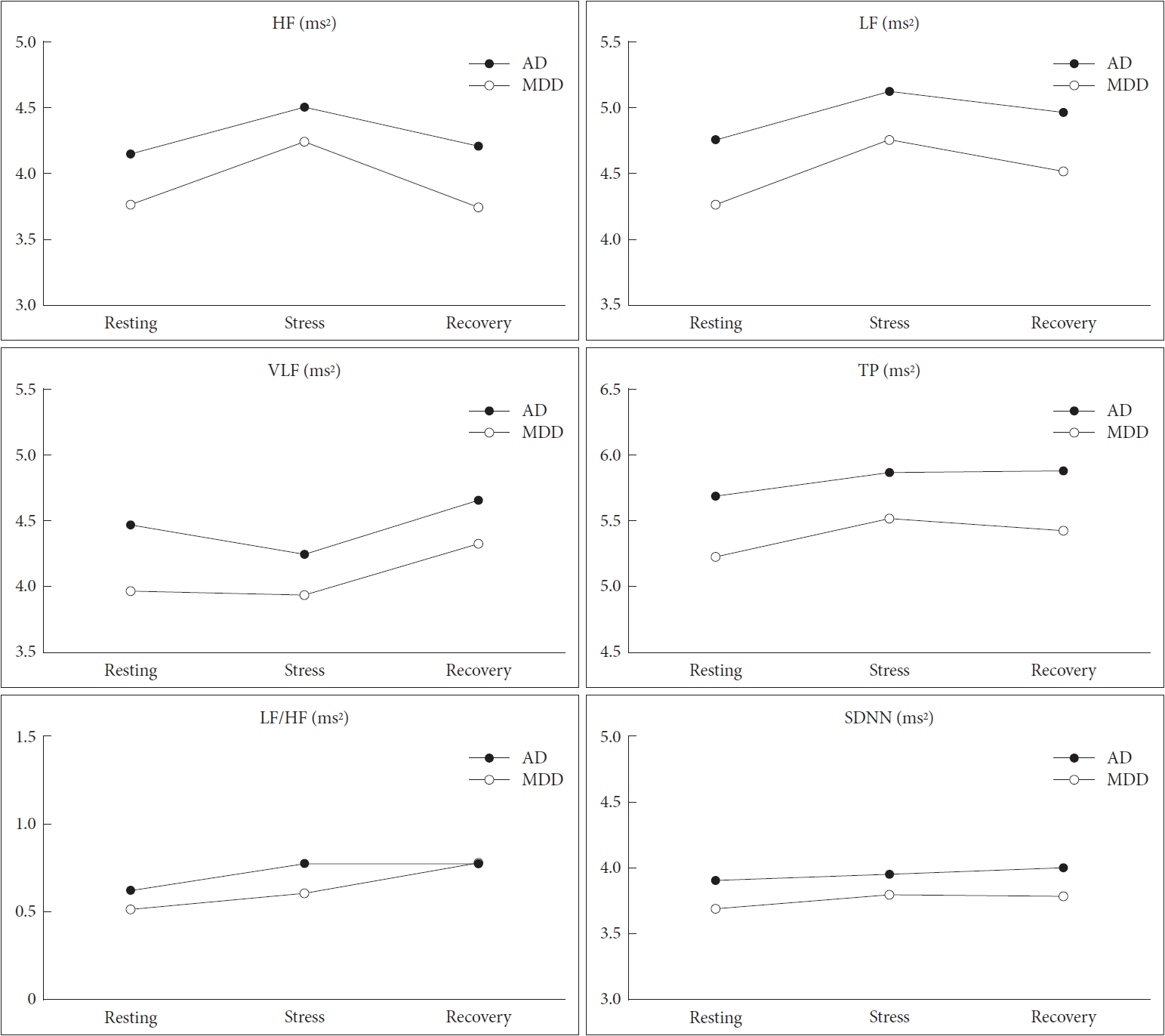
Comparison of reactive heart rate variability between adjustment disorder and major depressive disorder. All variables were logarithmically transformed due to their skewed distribution. AD: adjustment disorder, MDD: major depressive disorder, HF: high frequency band (ms2), LF: low frequency band (ms2), VLF: very low frequency band (ms2), TP: total power (ms2), LF/HF: ratio of LF to HF power, SDNN: standard deviation of normal-to-normal interbeat intervals (ms).
Although we found no intergroup differences in reactivity trends, Student’s t-tests revealed significant intergroup differences in the values of several HRV parameters (Figure 1, Supplementary Table 1 in the online-only Data Supplement). During the resting phase, the AD group showed higher LF (4.76± 0.96 ms2 vs. 4.27±1.12 ms2, p=0.016), VLF (4.47±0.85 ms2 vs. 3.97±0.93 ms2, p=0.004), TP (5.68±0.91 ms2 vs. 5.22±1.07 ms2, p=0.016), and SDNN (3.90±0.45 ms vs. 3.69±0.57 ms, p=0.038) when compared to MDD group. During the stress phase, the AD group only showed higher values for LF (5.13±0.87 ms2 vs. 4.76±0.78 ms2, p=0.022) and TP (5.86±0.85 ms2 vs. 5.51±0.77 ms2, p=0.030). Finally, LF (4.97±0.89 ms2 vs. 4.52±1.13 ms2, p=0.020), TP (5.87±0.92 ms2 vs. 5.42±1.07 ms2, p=0.021), and SDNN (4.00±0.47 ms2 vs. 3.78±0.52 ms2, p=0.021) were higher among the AD group than the MDD group during the recovery phase.
In correlation analyses, the changes of HRV parameters were correlated with symptom severity in AD whereas those correlations were less obvious in MDD. During the stress phase in AD group, the increase of HF had positive correlation with the scores of CES-D (r=0.468, p<0.001), PSS (r=0.413, p=0.001), and BAI (r=0.459, p<0.001), whereas the decreases of LF and LF/HF had correlation with increasing scores of BAI (r=-0.275, p=0.032) and PSS (r=-0.258, p=0.047) respectively. During the stress phase in MDD group, the decrease of SDNN had correlation with increasing scores of BAI (r=-0.291, p=0.047).
DISCUSSION
This study found no differences in the reactive trends among any HRV parameters during mental stress tasks between participants with AD and MDD. These results did not support our initial hypothesis. Rather, there were differences between the AD and MDD groups in HRV values such as SDNN, TP, LF, and VLF throughout whole phases. Increased focus should thus be placed on the quantitative aspects of autonomic activity rather than its reactive pattern to stresses when attempting to biologically distinguish AD from MDD.
MDD patients have consistently exhibited altered HRV reactivity during stress. This is especially true for HF (a proxy of parasympathetic activity [31]), which has thoroughly been replicated as a strong hallmark of MDD. In contrast to the normal stress reaction accompanied by sympathetic activation and parasympathetic withdrawal [32], HF further decreased or reversely increased in MDD patients during stress [33]. AD patients also showed smaller reactivity for HF during stress tasks than the healthy control group in our previous study [23]. This study was the first to compare autonomic reactivity between patients with AD and MDD. We replicated the increasing trends of HF during stress in AD patients as much as in those with MDD. We therefore speculate that AD shares a common pathophysiology with MDD as presented through an impaired parasympathetic withdrawal to the applied stress. Based on the significant correlation between HF changes and symptom severity in AD group of this study, we may suggest that the impaired parasympathetic withdrawal is one of the core biological marker of AD. However, as this study has a key limitation of lacking a direct comparison with healthy controls, we should be cautious to conclude that normal parasympathetic withdrawal was impaired in AD and MDD. Further studies including the data from healthy controls should follow to clearly understand the patterns of autonomic reactivity in both conditions.
Consistent with the results of HF, LF increased during stress tasks among patients with AD and MDD. Previous reports from healthy controls showed conflicting results for LF; some reported increasing LF [34,35], while others reported decreasing LF during stress tasks [36,37]. Reports from tests among MDD patients also revealed mixed results for the reactive change of LF to the applied stress [33]. Contrary to cases involving HF, there is thus a clear limitation to interpreting the reactive trends of LF revealed in this study. Notably, these results instead clarify the larger values of LF in AD patients more so than those in MDD patients during whole phases. LF could reflect both sympathetic and parasympathetic activity for short-term measurements such as those taken during this study [38,39]. Despite similar reactive patterns, patients with AD may therefore have greater overall autonomic activity compared to those with MDD.
AD patients had larger values of SDNN and TP than MDD patients during nearly all phases. Like LF, SDNN and TP have also been known to represent overall autonomic activity [40]. This evidence supports the idea of a more enhanced basal activity for the whole autonomic nervous system in AD patients when compared to those with MDD. Meanwhile, the reactive trends of SDNN and TP did not differ between AD and MDD patients; those variables increased during stress among participants with both conditions. Although the reactive change of TP to the stress was not well known, SDNN exhibited decreasing trends during stress tasks for normal stress reactions [32,37]. Liang et al. [41] reported decreasing SDNN and TP during mental arithmetic tasks in patients with MDD, which is contrary to this study’s findings. This discrepancy may be a result of differences in age, body mass index, antidepressant usage, and depressive episode histories between study participants.
We found decreasing trends for VLF during stress in both the AD and MDD groups. This was consistent with our previous report, which revealed decreasing VLF in AD patients and oppositely increasing VLF in healthy controls during stress tasks [23]. The VLF component mainly reflects sympathetic activity [39]. Thus, the impaired sympathetic dominance during stress may underlie both AD and MDD. Additionally, a larger resting VLF among AD patients when compared to MDD patients in this study may mean that AD patients have more enhanced basal sympathetic activity. However, our VLF results should be cautiously interpreted; the LF/HF ratio, which represents sympatho-vagal balance [39], was similar for both conditions. Furthermore, the relatively reduced VLF in MDD patients may reflect another pathophysiology rather than autonomic activity (e.g., inflammation) because VLF had a negative correlation with pro-inflammatory markers [42,43].
This study had several limitations. First, we could not exclude the influence of antidepressants; many participants had already been prescribed in an outpatient setting before admission. However, a meta-analysis revealed that antidepressants other than tricyclic agents had no significant effects on HRV measurement results [44]. Although other studies have reported opposite results [45,46], their focus was on long-term usage (e.g., about two years). This study excluded participants who were using tricyclic antidepressants. Furthermore, the mean duration of antidepressant usage was as short as six weeks. In addition, the impact of antidepressants on our findings did not seem significant because the mean duration of antidepressant usage and instances of significant antidepressant usage above four weeks were all comparable between the AD and MDD groups. Second, the neutral stress tasks used in this study could not directly reflect the nature of real-world stressors for AD patients. Third, there is a limitation of generalizing the results as the participants were composed of military soldiers only. Fourth, there was no information on the duration of disease which could lead to the substantial impact on HRV parameters. Finally, almost all HRV parameters used in this study were restricted to frequency-domain analyses due to our short-term measurement periods of five minutes. Further investigation is needed to determine whether autonomic reactivity varies according to the type of stressor and the duration of measurement.
Despite these limitations, this study was the first to compare the biological underpinnings of AD with those of MDD. There were no differences in reactive HRV trends regarding stress between the AD and MDD groups, which also had similar sociodemographic and clinical variables (including symptom severity). However, higher HRV values indicated higher basal activity in the autonomic nervous system for patients with AD compared to those with MDD. HRV may indicate the “allostatic capacity” for achieving adaptation through cardiac autonomic regulation during stress [47]. We thus suggest that AD patients may have a much better capacity for adapting to stressful conditions. This could partially explain the better prognoses and self-limiting disease courses of AD patients when compared to those among MDD patients, whose cardiac autonomic regulation is much more seriously dampened and inflexible.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.30773/pi.2020.0209.
Acknowledgements
This work was supported by a grant from the Armed Forces Medical Research Institute (Grant No. AFMC-12-012).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Dae Jong Oh, Eun Young Kim, Myung Jae Baik. Data curation: Dae Jong Oh, Sae Rom Kim, Myung Jae Baik. Formal analysis: Dae Jong Oh. Funding acquisition: Myung Jae Baik. Investigation: Dae Jong Oh, Eun Young Kim, Myung Jae Baik. Methodology: Dae Jong Oh, Eun Young Kim, Myung Jae Baik. Project administration: Eun Young Kim, Myung Jae Baik. Resources: Eun Young Kim, Myung Jae Baik. Software: Sae Rom Kim. Supervision: Myung Jae Baik. Validation: Eun Young Kim. Visualization: Dae Jong Oh. Writing—original draft: Dae Jong Oh. Writing—review & editing: all authors.

